- » Aim and Scope
- » Section Policies
- » Publication Frequency
- » Open Access Policy
- » Archiving
- » Peer-Review
- » Indexation
- » Publishing Ethics
- » Founder
- » Publication fee
- » Disclosure and Conflict of Interest
- » Plagiarism Policy
- » Preprint and postprint Policy
- » CrossMark Policy
- » Correction and retraction policy
- » Online First publication
- » Human and animal rights policy
- » Advertising policy
- » Data Sharing Policy
- » Your Paper, Your Way
- » Use of artificial intelligence in the preparation of an article
- » Informed Consent Policy
Aim and Scope
Sechenov Medical Journal is the official publication of the Sechenov University, one of the most famous medical schools in Russia.
Information about the structure, activities and focus of the Sechenov University is available on the website https://www.sechenov.ru/eng/.
The mission of the Journal is to provide an open platform for bringing together researchers, experts and practitioners in various fields of clinical and fundamental medicine to answer the most pressing questions and challenges of our time in the field of medicine.
The Sechenov Medical Journal aims to provide readers in Russia and abroad with open free information on topical issues of both fundamental - like cell biology, pathophysiology, biomedicine - and clinical sciences such as obstetrics and gynaecology, oncology, surgery, neurosurgery, internal medicine along with the best clinical practices.
Objectives:
- provide readers with quality content via careful selection of articles, carefully considering the interest and relevance of the topic, the originality and novelty of the proposed ideas, the reliability of the materials presented;
- provide researchers (authors) transparent publication process;
- adherence to international standards of publication ethics for all participants and at all stages of the publication process;
- implement modern publishing practices, including open publication of peer-reviews (Electronic Scientific Library (elibrary.ru)), publication of checklists for original studies and clinical cases (Equator-network), online first publications;
- attract and support young researchers in producing of high-quality scientific papers by the publication of educationalarticles on biomedical statistics and research design;
- create special issues on current topics of the journal with the involvement of the most significant international experts;
- respond to the global challenges of our time.
Our content is targeted at a wide range of researchers, specialists, general practitioners and family physicians. Our special focus is on young researchers and residents whom we see as the future of science and medicine.
The journal publishes original, experimental, pilot studies, reviews, clinical cases and unique clinical practices (How I Do It): the best scientific data of famous scientists from all the world, keeping up the expansion of the audience.
We aim to develop an interdisciplinary approach because of the unique scope of the journal's specialities: from fundamental to clinical sciences and publications in biomedicine (from biotechnology to medicine).
We are expanding our international audience reach, so in 2021 we moved from publishing metadata in English to publishing 50% of full-text articles in English. The journal is completely open to English-language manuscripts, and we see our task in further expanding the English-language content.
Section Policies
Publication Frequency
4 issues per year
Open Access Policy
Sechenov Medical Journal is an open access journal. All articles are made freely available to readers immediatly upon publication.
Our open access policy is in accordance with the Budapest Open Access Initiative (BOAI) definition - it means that articles have free availability on the public internet, permitting any users to read, download, copy, distribute, print, search, or link to the full texts of these articles, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose, without financial, legal, or technical barriers other than those inseparable from gaining access to the internet itself.
For more information please read BOAI statement.
Archiving
- Russian State Library (RSL)
- National Electronic-Information Consortium (NEICON)
- Portico digital preservation service
Peer-Review
This topic was last updated: July 09, 2025
Evaluating a scientific manuscript in Sechenov Medical Journal is carried out in several steps.
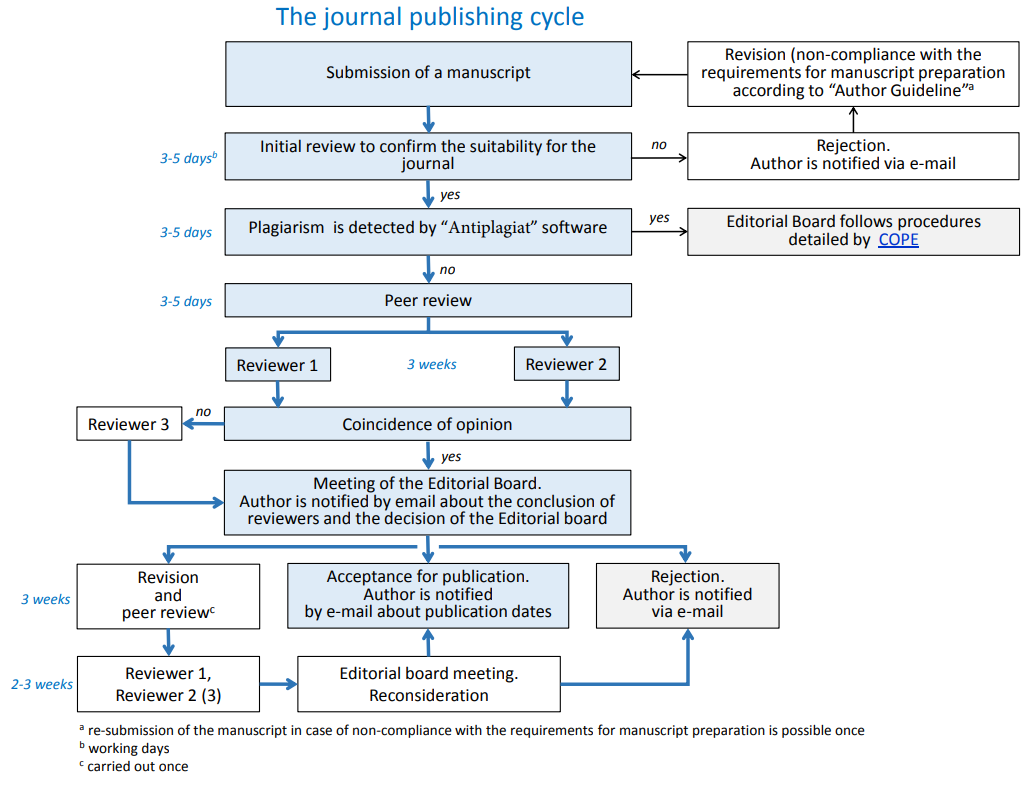
A total of 94% of all manuscripts submitted to the journal were unsolicited. Of these, about 20% were accepted for publication.
The average manuscript turnaround time of the serial publication is 16 weeks.
1. Initial review of the manuscript to comply with the standards of the Journal.
The scientific editor, the managing editor or the executive secretary provide an initial review of the manuscript within 3-5 days after its submission.
The initial review assesses the compliance of the manuscript with the scope and the standards of the journal on the basis of the checklist and the sample of the manuscript presented in the section "Author Guidelines".
If the manuscript does not meet the standards of the Journal or is out of its scope, it is rejected. Resubmission of the manuscript is possible after bringing it into line with the requirements of the journal.
All manuscripts are checked for plagiarism using the anti-plagiarism program “AntiPlagiat”. If scientific misconduct is alleged, the editor will follow procedures detailed by the Committee on Publication Ethics (COPE) and listed on the journal's website under plagiarism policy.
Manuscripts that are within the scope, meet the standards of the Journal and do not contain incorrect borrowing will be sent to reviewers.
2. Peer-review
A double-blind peer review method is mandatory for the processing of all submitted scientific manuscripts in Sechenov Medical Journal. This implies that neither the reviewer is aware of the authorship of the manuscript, nor the author maintains any contact with the reviewer.
Letters to the editor and editorials are not subject to peer review.
Each manuscript is peer-reviewed by 2 reviewers. In case of disagreement between the first reviewers, an additional 3rd reviewer is assigned.
We aim to limit the review process to 3-4 weeks, though in some cases the schedule may be extended by two weeks at the request of the reviewer.
Members of the Editorial Board or the Editorial Council and leading Russian and international experts in corresponding areas of medicine are invited to perform double-blind peer reviews. The Deputy editor-in-chief or the Science editor choose a reviewer for peer review. The reviewer must have publications for the last 3 years on the subject of the reviewed article. When choosing a reviewer, the editorial board takes into account potential conflicts of interest, so the reviewer cannot be the supervisor, subordinate, or employer of the author, co-author of previous articles, a relative of the author.
For articles where the authors are the editor-in-chief, deputy editor-in-chief, scientific editor, members of the editorial board or the editorial board of the journal, reviewing is necessarily carried out only by external reviewers. Editor delegates handling the peer reviewer of any of their own work submitted to the journal (excluding editorials) to another editor of the journal.
Each reviewer has the right to refuse to do a review if there is any conflict of interest affecting the perception and interpretation of the manuscript. Reviewing is voluntary and free of charge.
The transparency in the publication process is a key principle for the Editorial Board of the Sechenov Medical Journal. When a manuscript is published, the full text of the reviews is published on the journal website with the article in a separate tab without specifying reviewer data. The reviews are also transferred to the Russian Index of Science Citation - RISC (elibrary) database together with the article text. The personal data of reviewers will be hidden from all RISC users, and their personal profiles will reflect their contributions to the work on articles in our journal.
Reviewers should follow the Sechenov Medical Journal’s Publication Ethics based on international recommendations (The Committee on Publication Ethics (COPE), the Directory of Open Access Journals (DOAJ), the Open Access Scholarly Publishers Association ( OASPA), the World Association of Medical Editors (WAME), Association of Scientific Editors and Publishers (ANRI)).
The reviewer should:
I. Critically evaluate the manuscript, while remaining constructive in their comments and prepare detailed comments on the study and manuscripts to help the authors improve the work. Examination of the work should include an assessment of the originality and significance of the study; study design; research methodologies, including analytical and statistical methods; presentation of the results; the validity of conclusions. In addition, it is necessary to identify possible distortions and errors and evaluate the overall quality of the manuscript.
II. Provide the editor with recommendations on the advisability of publishing the manuscript in this journal.
III. The Reviewer must inform the editor of a potential conflict of interest that may arise in relation to the authors or the content of the manuscript that is proposed for review. In most cases, when a conflict of interest arises, the Reviewer has the right to refuse to review.
IV. The Reviewer must guarantee the confidentiality of information contained in the manuscript, and should not use this information in any way.
V. Double-blind peer review implies that the reviewer receives the manuscript without the personal data of the authors, and the authors receive the opinion of the Reviewer without the personal data of the Reviewer.
The review is compiled in the standard form proposed by the editor for each type of manuscript, with the mandatory coverage of the following provisions:
- subject area compliance
- relevance and scientific novelty
- practical relevance
- ethics
- the validity of design, research methods, structure and content
- quality of article design: style, terminology and its conformity to the accepted in the field of knowledge
Based on the manuscript review, recommendations provided by the reviewer should be one of the following types (each decision of the reviewer is justified):
- accept submission
- minor revision
- major revision and review
- decline submission
To accept the paper in its present state
The manuscript is ready for publication in its current submission, substantiated, ethical, significant for the scientific community, the writing style is clear and concise.
To accept after a minor revision
There are uncritical comments on the manuscript that need to be corrected.
This may be a bad style, lack of clarity of presentation, insufficiently developed structure, errors in links, duplication of information in figures and tables and in the text of the manuscript. After changes and reassessment, the manuscript can be accepted for publication.
Major revision and subsequent review
The article has serious flaws and errors that affect the reliability of the results: problems with ethics, research design, gaps in the description of research methods, poorly presented results or their misinterpretation, an insufficiently complete description of the limitations of the study, contradictory (or disproved by the author’s own statements) conclusions, lack of references to important studies, fuzzy tables and figures requiring serious revision.
After a subsequent review, the manuscript can be accepted, rejected or sent for additional review.
Time to review manuscript after revision - 2-3 weeks.
To decline the manuscript
The work does not meet the scope and aims of the journal, has one or more irreparable defects or serious ethical problems: consent for publication was not obtained in cases where it is necessary, the research methods are unethical, the methodology is discredited or erroneous (for example, a process that seriously affects results).
The reviewer should give detailed comments, as they can help the author significantly improve the work.
3. Revisions, final submission, and acceptance
In cases where the review has requested changes to the manuscript, authors will be invited to prepare a revision. The decision letter from the editorial office will be sent to the authors. The revision should also be accompanied by a point-by-point response to referees explaining how the manuscript has been changed. The deadline for submission of a revised manuscript is 3 weeks.
We kindly request to notify the editor in writing if the author decides to refuse to publish the manuscript.
If the author and reviewers have encountered insoluble contradictions regarding the manuscript, the editorial board has the right to send the manuscript for additional review. The duration of the additional review is 2-3 weeks. In conflict situations, a decision is made at a meeting of the Editorial Board.
Kindly note that a positive review does not guarantee acceptance, as the final decision in all cases is made by the Editorial board.
The decision to refuse to publish the manuscript is made at a meeting of the Editorial Board in accordance with the recommendations of reviewers. A manuscript rejected by the Editorial Board is not accepted for reconsideration. A reasoned refusal to publish is sent to the author by e-mail and includes copies of the texts of the conclusions of the reviewers (scientific editor) and the decision of the editorial board.
Upon the decision to accept the manuscript for publishing, the editorial office notifies the authors of the scheduled date of publication.
Reviews are kept in the editorial for 5 years and can be transferred to the Higher Attestation Commission upon request.
Indexation
Articles in "Sechenov Medical Journal" are indexed by several systems:
- Russian Index for Science Citation (RISC) – a database, accumulating information on papers by Russian scientists, published in native and foreign titles. The RSCI project is under development since 2005 by the “Electronic Scientific Library” foundation (elibrary.ru).
- Russian Science Citation Index (RSCI) on the Web of Science platform - joint project of the Russian Academy of Sciences, Clarivate Analytics and Scientific Electronic Library eLIBRARY.RU
- Google Scholar is a freely accessible web search engine that indexes the full text of scholarly literature across an array of publishing formats and disciplines. The Google Scholar index includes most peer-reviewed online journals of Europe and America's largest scholarly publishers, plus scholarly books and other non-peer-reviewed journals.
- EBSCO, one of the world's largest content aggregators and research database providers, helps link the content in the Journal into the research literature.
- Ulrichsweb is the authoritative source of bibliographic and publisher information on more than 300,00 periodicals of all types of academic and scholarly journals, Open Access publications, peer-reviewed titles, popular magazines, newspapers, newsletters, and more from around the world. It covers all subjects and includes publications that are published regularly or irregularly and that are circulated free of charge or by paid subscription.
- Directory of Open Access Journals (DOAJ)
Publishing Ethics
The Publication Ethics and Publication Malpractice Statement of the Sechenov Medical Journal is based on the principles of transparency and best practice for scholarly publications suggested by The Committee on Publication Ethics (COPE), the Directory of Open Access Journals (DOAJ), the Open Access Scholarly Publishers Association (OASPA), and the World Association of Medical Editors (WAME) in collaboration can be accessed here https://publicationethics.org/resources/guidelines-new/principles-transparency-and-best-practice-scholarly-publishing) and the recommendations of the International Committee of Medical Journal Editors (ICMJE) Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals and requirements for peer-reviewed medical journals (https://www.elsevier.com/__data/assets/pdf_file/0018/116082/Publication-Ethics-and-Malpractice-Statement-requirementsScopusJune-2021.pdf), elaborated by the Elsevier Publishing House (in accordance with international ethical rules of scientific publications)
The Sechenov Medical Journal strictly adheres to the principle of editorial independence, defined as the clear separation of editorial decision-making from commercial, financial, or ownership interests. Our editorial policy is informed by the World Association of Medical Editors' policy on the relationship between journal editors-in-chief and owners, and by RELX's editorial standards policy, to which we comply.
1. Introduction
1.1. The publication in a peer-reviewed learned journal serves many purposes outside of simple communication. It is a building block in the development of a coherent and respected network of knowledge. For all these reasons and more it is important to lay down standards of expected ethical behavior by all parties involved in publishing: the Author, the journal Editor, the Peer Reviewer, the Publisher and the society for society-owned or sponsored Journal: “Sechenov Medical Journal”.
1.2. The Publisher has a supporting, investing, and nurturing role in the scholarly communication process but is also ultimately responsible for ensuring that best practice is followed in their publications.
1.3. The Publisher takes its duties of guardianship over the scholarly record extremely seriously. Our journal programs record «the minutes of science» and we recognize our responsibilities as the keeper of those «minutes» in all our policies not least the ethical guidelines that we have here adopted.
2. Duties of the Editors
2.1. Publication decision.
The Editor of the “Sechenov Medical Journal” is solely and independently responsible for deciding which of the articles submitted to the journal should be published, often working in conjunction with the relevant society (for society-owned or sponsored journals). The validation of the work in question and its importance to researchers and readers must always underwrite such decisions. The Editor may be guided by the policies of the “Sechenov Medical Journal”, the journal’s Editorial board, and constrained by such legal requirements which then will be in force regarding libel, copyright infringement, and plagiarism. The Editor may confer with other Editors or Reviewers (or society officers) in making this decision.
2.2. Fair play.
The Editor should evaluate manuscripts for their intellectual content without regard to race, gender, sexual orientation, religious belief, ethnic origin, citizenship, or political philosophy of the Authors.
2.3. Confidentiality.
The Editor and any Editorial staff of the “Sechenov Medical Journal” must not disclose any information about a submitted manuscript to anyone other than the corresponding Author, the Reviewers, potential Reviewers, other Editorial advisers, and the Publisher, as appropriate.
2.4. Disclosure and Conflicts of interest
2.4.1. Unpublished materials disclosed in a submitted manuscript must not be used in the Editor’s own research without the express written consent of the Author. Information or ideas obtained through peer review must be kept confidential and not used for personal advantage.
2.4.2. The Editors should recuse themselves (i.e. should ask a co-editor, associate editor or another member of the Editorial board instead to review and consider) from considering manuscripts in which they have conflicts of interest resulting from competitive, collaborative, or other relationships or connections with any of the authors, companies, or (possibly) institutions connected to the papers.
2.5. Vigilance over the published record.
The Editor presented with convincing evidence that the substance or conclusions of a published paper are erroneous should coordinate with the Publisher (and/or society) to promote the prompt publication of a correction, retraction, expression of concern, or another note, as may be relevant.
2.6. Involvement and cooperation in investigations.
The Editor should take reasonably responsive measures when ethical complaints have been presented concerning a submitted manuscript or published paper, in conjunction with the Publisher (or society). Such measures will generally include contacting the Author of the manuscript or paper and giving due consideration of the respective complaint or claims made but may also include further communications to the relevant institutions and research bodies.
3. Duties of the Reviewers
3.1. Contribution to Editorial Decisions.
Peer review assists the Editor in making editorial decisions and through the editorial communications with the Author may also assist the Author in improving the paper. Peer review is an essential component of formal scholarly communication and lies at the heart of the scientific method. Publisher shares the view of many that all scholars who wish to contribute to publications have an obligation to do a fair share of reviewing.
3.2. Promptness.
Any selected referee who feels unqualified to review the research reported in a manuscript or knows that its prompt review will be impossible should notify the Editor of the “Sechenov Medical Journal” and excuse themselves from the review process.
3.3. Confidentiality.
Any manuscripts received for review must be treated as confidential documents. They must not be shown to or discussed with others except those authorized by the Editor.
3.4. Standard and objectivity.
Reviews should be conducted objectively. Personal criticism of the author is inappropriate. The Referees should express their views clearly with supporting arguments.
3.5. Acknowledgement of Sources.
The Reviewers should identify relevant published work that has not been cited by the Authors. Any statement that an observation, derivation, or argument had been previously reported should be accompanied by the relevant citation. The Reviewer should also call to the Editor’s attention any substantial similarity or overlap between the manuscript under consideration and any other published paper of which they have personal knowledge.
3.6. Disclosure and Conflict of Interest.
3.6.1. Unpublished materials disclosed in a submitted manuscript must not be used in the Reviewer’s own research without the express written consent of the Author. Information or ideas obtained through peer review must be kept confidential and not used for personal advantage.
3.6.2. The Reviewers should not consider manuscripts in which they have conflicts of interest resulting from competitive, collaborative, or other relationships or connections with any of the authors, companies, or institutions connected to the papers.
4. Duties of the Authors
4.1. Reporting standards
4.1.1. The Authors of the original research should present an accurate account of the work performed as well as an objective discussion of its significance. Underlying data should be represented accurately in the paper. A paper should contain sufficient detail and references to permit others to replicate the work. Fraudulent or knowingly inaccurate statements constitute unethical behavior and are unacceptable.
4.1.2. Review and professional publication articles should also be accurate and objective, and editorial 'opinion’ works should be clearly identified as such.
4.2. Data Access and Retention – Authors may be asked to provide the raw data in connection with a paper for editorial review, and should be prepared to provide public access to such data (consistent with the ALPSP-STM Statement on Data and Databases), if practicable, and should, in any event, be prepared to retain such data for a reasonable time after publication.
4.3. Originality and Plagiarism
4.3.1. The authors should ensure that they have written entirely original works, and if the Authors have used the work and/or words of others, this has been appropriately cited or quoted.
4.3.2. Plagiarism takes many forms, from ‘passing off’ another’s paper as the Author’s own paper, to copying or paraphrasing substantial parts of another’s paper (without attribution), to claiming results from research conducted by others. Plagiarism in all its forms constitutes unethical publishing behavior and is unacceptable.
4.4. Multiple, Redundant or Concurrent Publication
4.4.1. An Author should not in general publish manuscripts describing essentially the same research in more than one journal of primary publication. Submitting the same manuscript to more than one journal concurrently constitutes unethical publishing behavior and is unacceptable.
4.4.2. In general, an author should not submit for consideration in another journal a previously published paper.
4.4.3. Publication of some kinds of articles (eg, clinical guidelines, translations) in more than one journal is sometimes justifiable, provided certain conditions are met. The Authors and the Editors of the journals concerned must agree to the secondary publication, which must reflect the same data and interpretation of the primary document. The primary reference must be cited in the secondary publication. Further detail on acceptable forms of secondary publication can be found at www.icmje.org.
4.5. Acknowledgement of Sources.
Proper acknowledgment of the work of others must always be given. The Authors should cite publications that have been influential in determining the nature of the reported work. Information obtained privately, as in conversation, correspondence, or discussion with third parties, must not be used or reported without explicit, written permission from the source. Information obtained in the course of confidential services, such as refereeing manuscripts or grant applications, must not be used without the explicit written permission of the Author of the work involved in these services.
4.6. Authorship of the Paper
4.6.1. Authorship should be limited to those who have made a significant contribution to the conception, design, execution, or interpretation of the reported study. All those who have made significant contributions should be listed as co-authors. Where there are others who have participated in certain substantive aspects of the research project, they should be acknowledged or listed as contributors.
4.6.2. The corresponding Author should ensure that all appropriate co-authors and no inappropriate co-authors are included on the paper, and that all co-Authors have seen and approved the final version of the paper and have agreed to its submission for publication.
4.7. Hazards and Human or Animal Subjects
4.7.1. If the work involves chemicals, procedures or equipment that have any unusual hazards inherent in their use, the author must clearly identify these in the manuscript.
4.7.2. If the work involves the use of animal or human subjects, the Author should ensure that the manuscript contains a statement that all procedures were performed in compliance with relevant laws and institutional guidelines and that the appropriate institutional committee(s) has approved them. The Authors should include a statement in the manuscript that informed consent was obtained for experimentation with human subjects. The privacy rights of human subjects must always be observed.
4.8. Disclosure and Conflicts of Interest
4.8.1. All Authors should disclose in their manuscript any financial or other substantive conflict of interest that might be construed to influence the results or interpretation of their manuscript. All sources of financial support for the project should be disclosed.
4.8.2. Examples of potential conflicts of interest which should be disclosed include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding. Potential conflicts of interest should be disclosed at the earliest possible stage.
4.9. Fundamental errors in published works.
When the Author discovers a significant error or inaccuracy in a published work, it is the Author’s obligation to promptly notify the Editor of “Sechenov Medical Journal” and cooperate with Publisher to retract or correct the paper. If the Editor or the Publisher learns from a third party that a published work contains a significant error, it is the obligation of the Author to promptly retract or correct the paper.
5. Duties of the Publisher (and if relevant, Society)
5.1. The Publisher should adopt policies and procedures that support the Editors, the Reviewers, and the Authors of “Sechenov Medical Journal” in performing their ethical duties under these ethics guidelines. The Publisher should ensure that the potential for advertising or reprint revenue has no impact or influence on editorial decisions.
5.2. The Publisher should support “Sechenov Medical Journal” Editors in the review of complaints raised concerning ethical issues and help communications with other journals and/or publishers where this is useful to the Editors.
5.3. The Publisher should develop codes of practice and inculcate industry standards for best practice on ethical matters, errors and retractions.
5.4. The Publisher should provide specialized legal review and counsel if necessary.
Founder
- Federal State Autonomous Educational Institution of Нigher Education I.M.Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenovskiy University)
The Sechenov Medical Journal is funded by the founder.
Publication fee
- Publication in Sechenov Medical Journal is free for authors.
- The Editors do not charge authors for the preparation, placement, and printing the materials.
Disclosure and Conflict of Interest
All participants in the peer-review and publication process should consider and disclose all relationships that could be a potential source of conflict of interest (financial relationships (e.g. employment, consulting, shareholding, patents, or paid peer review), personal relationships, academic competition, and intellectual convictions).
a) Authors
All authors have to disclose all financial and personal relationships that influenced or could affect their work, indicating it in the Copyright Agreement and in the text of the manuscript:
- The presence or absence of a conflict of interest for all authors; and
- Sources of support for this work (funding), including sponsors, if any, - also an explanation of their role in developing the research plan; collection, analysis, and interpretation of data; drawing up a report; deciding on submitting a report for publication; or a statement that the source of support was not involved;
b) Reviewers
Reviewers should not consider manuscripts in which they have conflicts of interest resulting from competitive, collaborative, or other relationships or connections with any of the authors, companies, or institutions connected to the papers.
c) Journal Editors and Staff
Journal Editors and Staff should not consider manuscripts and make editorial decisions when there is a conflict of interest or relationship that may create a conflict of interest.
Unpublished materials disclosed in a submitted manuscript must not be used in a Reviewer’s own research without the express written consent of the Author. Information or ideas obtained through peer review must be kept confidential and not used for personal advantage.
Plagiarism Policy
By submitting a manuscript to the Sechenov Medical Journal, authors certify that all submitted material, including text, figures, tables, raw data, and other accompanying files, is their original work, has been prepared independently, and is not simultaneously under review or submission to other journals.
The editorial team checks all incoming materials with the help of the Russian text plagiarism detection system "Antiplagiat" (https://www.antiplagiat.ru ). Since November 2024, this system has a module "Duplicate Detector", which records cases when an identical or very similar document was previously checked by the system in another organization. In such a case, the mark "checked in another organization" appears in the report (https://antiplagiat.ru/detector-faq/).
The Editorial Office takes into account that the manuscript submitted by the authors may have been previously reviewed by another journal and not accepted for consideration for various reasons. In such cases, the authors are obliged to attach to the manuscript information about the fact of rejection, preferably in the form of an official letter from the Editorial Office of the corresponding journal. In the absence of such information, the Editorial Office has the right to request a written explanation. Failure to provide such an explanation may result in rejection of the manuscript without further consideration. The Editorial Office considers simultaneous submission and duplication of publications unacceptable.
When reviewing a manuscript, the editorial team will check the text materials in Russian and English using the Anti-Plagiarism System for illegal borrowings, incorrect borrowings, unfair self-citations, lack of necessary self-citations, signs of borrowings masking (including rewriting and attempts to circumvent the system), signs of generated text, translated borrowings (including machine and free translation). The check is performed by certified experts who have undergone advanced training in the use of the anti-plagiarism system.
For all illustrative materials (graphics, images, diagrams), the editorial team performs manual checks to detect illegal borrowing, incorrect borrowing, unfair self-citations, lack of required self-citations, and signs of image manipulation.
If violations of publication ethics are found, the Editorial Board acts in accordance with the rules of the Committee on Publication Ethics (COPE).
Preprint and postprint Policy
Prior to acceptance and publication in "Sechenov Medical Journal", authors may make their submissions available as preprints on personal or public websites.
As part of the submission process, authors must declare and link to any preprint publication and confirm that the submission has not been previously published or submitted to another journal.
After a manuscript has been published in "Sechenov Medical Journal" we suggest that the link to the article on the journal's website is used when the article is shared on personal or public websites.
CrossMark Policy
CrossMark is a multi-publisher initiative from Crossref, provides a standard way for readers to locate the authoritative version of an article or other published content. By applying the CrossMark logo, journal "Sechenov Medical Journal" is committing to maintaining the content it publishes and to alerting readers to changes if and when they occur.
Clicking the CrossMark logo on a document will tell you its current status and may also give you additional publication-record information about the document.
Correction and retraction policy
Changes in the article accepted for publication, which went through the stages of peer review and prepress (publication online first is included), fall into one of three categories:
- Addendum,
- Publisher Correction (Erratum),
- Author Correction (Corrigendum).
Decisions about types of correction are made by the journal's editors, sometimes with the advice of reviewers, Editorial Council or Editorial Board Members. This process involves consultation with the authors of the paper, but the editors/ Editorial Board Members make the final decision about whether an amendment is required and the category in which the amendment is published.
- Addendum.
Adding new material to the accepted article that supplements its original content (addendum) requires mandatory reviewing. Additional material is uploaded on the journal's website as a new manuscript with a link to the original article.
If the new material should replace the original content of the accepted article, the editor may consider the publication of an erratum or a corrigendum. - Publisher Corrections (erratum) is published in case of an error (typo, missed change) introduced by the journal in production, which is significant and affects the understanding of the article by the reader. Corrections are not published for simple, obvious typographical errors.
- Author Corrections (Corrigendum). If the authors consider it necessary to make corrections after the publication of the article (corrigendum), it is necessary to send a written (by email) request with justification to the editorial office of the journal. The final decision on the publication of the correction (corrigendum) is made by the editors of the journal and members of the Editorial Board after assessing the impact of the change on the scientific accuracy and significance of the published article. In some cases, the identification of serious errors and inconsistencies in the published article may require retraction of the article.
Retraction policy
According to the recommendations of Committee on Publication Ethics (COPE Council. COPE Retraction guidelines — English. https://doi.org/10.24318/cope.2019.1.4 ), the withdrawal of the text from the publication (retraction) is possible to correct the published information and notify readers that the publication contains serious flaws or erroneous data that cannot be trusted. Data inaccuracy may result from misconception or deliberate breach.
Retraction is also used to warn readers about cases of redundant publication, plagiarism, peer review manipulation, reuse of material or data without authorization, copyright infringement or some other legal issue (eg, libel, privacy, illegality), unethical research, and/or a failure to disclose a major competing interest that would have unduly influenced interpretations or recommendations.
According to COPE Retraction Guidelines, Editors of the Sechenov Medical Journal consider retracting a publication if:
- Clear evidence that the findings are unreliable, either as a result of a major error (eg, miscalculation or experimental error) or as a result of fabrication (eg, of data) or falsification (eg, image manipulation)
- Detection of incorrect borrowings (plagiarism) in the publication;
- The findings have previously been published elsewhere without proper attribution to previous sources or disclosure to the editor, permission to republish, or justification (ie, cases of redundant publication)
- It contains material or data without authorization for use
- copyright has been infringed or there is some other serious legal issue (eg, libel, privacy)
- It reports unethical research
- It has been published solely on the basis of a compromised or manipulated peer review process
- The author(s) failed to disclose a major competing interest (aka, conflict of interest) that, in the view of the editor, would have unduly affected interpretations of the work or recommendations by editors and peer reviewers.
If the author/group of authors find it necessary to withdraw the article, they contact the editorial office, explaining the reason for their decision. If the editorial board agrees to retraction, then it independently retracts the text.
If the editorial board decides to withdraw the text based on its expertise or information received by the editorial board, the author/group of authors is informed of this decision, with a justification for retraction of the article. If the author/team ignores the editorial request, it is appropriate to seek assistance from the Council on the Ethics of Scientific Publications.
Having decided to withdraw the article, the editors indicate the reason for the retraction (if plagiarism is found, indicating the sources of borrowing), as well as the date of retraction. The article and the description of the article remain on the journal's website as part of the corresponding issue of the journal, but the inscription WITHDRAWAL / RETRACTED and the retraction date are applied to the electronic version of the text, the same mark is placed with the article in the table of contents of the issue.
The Council for the Ethics of Scientific Publications and the Scientific Information Base (NEB, CyberLeninka) is provided with a protocol, which indicates the date of the meeting, the composition of the meeting, the results of the examination, a reasoned decision, and a completed form.
Online First publication
We publish online ahead of print in the Online First section on the website of the journal articles that have passed reviewing, editing, and proofreading and accepted for publication, text, and layout of which are agreed with all authors.
The article published Online First is the final version. Corrections to the article published in the "Online first" section can be made only as an Addendum, Erratum, or Corrigendum in the next issue of the journal.
You can cite an article Online First, indicating the name of the author, the title of the article, the title of the journal, the year of publication, and the DOI.
After the printed version of the issue of the journal is released, the article is moved from the Online First section to the Current Issue.
Human and animal rights policy
All research submitted to Sechenov Medical Journal for consideration must have been conducted in accordance with the international fundamental principles of the research ethics of studies involving humans and animals. In accordance with the recommendations of the international Committee on Publication Ethics (COPE), the editors reserve the right to reject any manuscript that, in the opinion of the editors, does not meet high ethical standards.
Studies Involving Human Subjects
Research involving humans must be conducted in accordance with the Declaration of Helsinki of the World Medical Association, with the prior approval of the relevant local ethics committee and the informed written consent of all human subjects participating in the research. Patients may be included in a scientific study only after they have received full information about it and have given their informed and voluntary consent to participate. Research involving subjects physically or mentally incapable of consenting, such as unconscious patients, may only be conducted if a physical or mental condition that precludes obtaining informed consent is an inherent characteristic of the study population. In such cases, the physician should seek informed consent from the legal representative.
Additional information and documentation to support this should be made available to the editor upon request. Manuscripts may be rejected if the editor considers that the research was not conducted within an appropriate ethical framework. On rare occasions, the Editor may contact the Ethics Committee for additional information.
Authors should include the following statement in the text of the manuscript:
“The study was approved by the Ethics Committee (full name and affiliation of the Ethics Committee, protocol number and date) and was conducted in accordance with the Helsinki Declaration of Human Rights. Patients/participants provided written informed consent to participate in this study.
Consent to publish
Authors should make sure to receive consent from the patients to publish their data prior to submitting their paper to a journal. This is in particular applicable to case studies.
Consent from the patient may be obtained in a form accepted by the institution or local Ethics Committee and must clearly state how the patient's identifiable information will be used. You can use the consent form provided on the Sechenov Medical Journal website.
The written consent of the patient should be available at the request of the editors during the review of the manuscript, as well as after publication. Manuscripts may be rejected if the required patient consent form cannot be provided.
See also section on Informed Consent Policy.
Studies Involving Animal Subjects
Experimental animal studies must comply with local and international standards for the use of laboratory animals and must be approved by the appropriate Ethics Committee. The Basel Declaration and the recommendations of the ICLAS (International Council for Laboratory Animal Science) set out the basic principles to be followed when conducting research on animals.
A statement stating that the relevant guidelines have been followed and that Ethics Committee approval has been obtained (including the name of the Ethics Committee, protocol number and date) must be included in the manuscript.
Sechenov Medical journal encourages authors to follow the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) for the design, analysis, and reporting of scientific research involving animals.
Advertising policy
The main principles of the Sechenov Medical Journal advertising policy presented in this section are based on the recommendations of the World Association of Medical Editors (WAME)
Advertisers and sponsors have no control over the editor's decisions, regardless of the terms of advertising or other agreements. The journal has the right to refuse to place any advertisement for any reason. The decision to publish an advertisement can only be made with the participation of the editorial team and the consultation with editor-in-chief and deputy editor-in chief of the journal. The content of scheduled issues and special supplementary issues is a subject to the sole discretion of the editor and is not affected by sponsors or advertisers.
Advertised products must be oriented towards medical practice, medical education or medical care. All advertisements uniquely identify the advertiser and the product or service being offered. Drug advertisements must include the full name of each active ingredient.
Promotional content must be distinguished from editorials and articles and highlighted so that the difference between them is obvious. Commercial advertisements must not be placed adjacent to any editorial or article that discusses the advertised product, nor should they contain references to the issue of the journal in which they appear.
Advertising placed in the journal must not deceive or mislead. Advertising must not contain offensive language of a racial, religious or other nature.
Data Sharing Policy
The Sechenov Medical Journal encourages authors to make the research data that support their publications available.
When reviewing an article, the editors of the Sechenov Medical Journal have the right to require authors to provide primary research data confirming the results presented in the article.
Definition of research data
Research data includes any recorded factual material that are used to produce the results in digital and non-digital form. This includes tabular data, code, images, audio, documents, video, maps, raw and/or processed data.
This policy applies to the research data that would be required to verify the results of research reported in articles published in the Sechenov Medical Journal. Research data include data produced by the authors (“primary data”) and data from other sources that are analysed by authors in their study (“secondary data”).
Data storage
The preferred mechanism for sharing research data is via data repositories. Another option for sharing data is to upload it as an additional file to the journal website along with the article. This option is possible for small data sets.
If you need help finding research data repositories, please refer to the list of repositories on the websites:
Data access statement
Authors should provide a data availability statement that will be published in their paper.
Below are standard templates for the «Data access» statement.
- «The data that support the findings of this study are openly available in [repository name] at [URL], reference number [reference number]».
- «The data that support the findings of this study have been published as supplementary files and available via link …»
- «The data that support the findings of this study are available from the corresponding author upon reasonable request».
Data citation
The Editorial Board of the Sechenov Medical Journal welcomes access to data under Creative Commons Licenses but does not insist on the obligatory use of Creative Commons in case when the data is deposited in the repositories of the third party. The Publisher of the Sechenov Medical Journal does not assert any copyrights for the data submitted by the author together with the article.
Questions regarding the observation of that policy shall be sent to the executive secretary of the Sechenov Medical Journal to the editorial email.
Your Paper, Your Way
10.11.2025
The Sechenov Medical journal has joined "Your Paper, Your Way" initiative, which simplifies the requirements for the format of a manuscript at the stage of submission to the journal.
You can submit your manuscript to the journal for initial consideration and peer review as a single Word file without special editing in any format along with the necessary documents (cover letter and checklist for the appropriate study design). Tables and figures are included in the text of the manuscript and should be accompanied by a legend. The manuscript must contain all the necessary components, according to its type. The size of the uploaded file containing the manuscript text must not exceed 100 MB.
Only when your manuscript moves to revision, you will be asked to bring it into the "journal format" and provide all the elements necessary for the publication.
The main requirements for submitting a manuscript according to the principle "Your paper - your way":
- Authors submit their entire manuscript, including text, figures and tables in one file
- Tables and figures must be of sufficiently high quality and have a legend
- The manuscript must contain the essential elements required for the appropriate type of manuscript
- Bibliographic references may be in any uniform style or format. The name(s) of the author(s), title of the journal/book, title of the article, year of publication, volume and issue/chapter of the book, pagination, DOI should be indicated. Authors will be asked to issue bibliographic references in the style adopted in the journal at the stage of finalizing the manuscript before publication
- When the manuscript is under revision and corrections after the successful completion of the review, the authors will be asked to provide all the elements that are necessary for publication, such as source files of graphics and images in an editable format
Use of artificial intelligence in the preparation of an article
In connection with the spreading practice of using programs based on artificial intelligence, including in the preparation and writing of scientific articles, the editorial board of the Sechenov Medical Journal considers it necessary to focus the authors' attention on the following provisions.
- Chat-bots such as ChatGPT (and similar ones) can under no circumstances be listed as the author of the article or a person who contributed to the preparation of the article. Artificial intelligence-based programs and tools do not meet the requirements of authorship because they are not (and cannot be) responsible for the research submitted, cannot declare the presence and absence of conflicts of interest, and cannot manage copyright.
- The use of chatbots or other artificial intelligence-based programs is NOT ALLOWED when writing the TEXT of the article and METADATA or for generating illustrations. In some cases, such programs may be useful for text editing, searching for additional sources of literature, data collection and analysis. However, it should be considered that chatbots often transmit false information to the user (literally "invent" non-existent facts and links to publications that never existed), so authors should check the information received from chatbots.
- If a program based on artificial intelligence was used for editing the text, searching for additional sources of literature, collecting and analyzing data, it is necessary to indicate this information in the article. Always indicate the version of the program and the date of use.
- Only the author is responsible for the final text of the article submitted to the Sechenov Medical Journal, regardless of which artificial intelligence-based programs and to what extent was used.
- The Editors of the Sechenov Medical Journal uses the Anti-Plagiarism module, which allows to detect the generated text.
- Editors and reviewers cannot share confidential manuscript information with the generative AI chatbot.
Sechenov Medical Journal shares the position of the international publishing community regarding the use of artificial intelligence in the preparation of scientific articles, as stated in the following documents: Chatbots, Generative AI, and Scholarly Manuscripts (WAME Recommendations on Chatbots and Generative Artificial Intelligence in Relation to Scholarly Publications); Artificial intelligence (AI) in decision making.
Informed Consent Policy
The Sechenov Medical Journal follows the recommendations of the International Committee of Medical Journal Editors (ICMJE) and the Committee on Publication Ethics (COPE), which states that patients/study participants have a right to privacy that cannot be violated without their consent.
The researchers and authors of the manuscript are responsible for respecting the principles of confidentiality of information relating to the health status and identity of patients. According to these recommendations, authors should exclude from the manuscript all non-essential details that allow identification of a person and should obtain written informed consent from the patient or their official representative for the publication of any potentially identifiable data or images.
Images such as x-rays, endoscopic images, ultrasound images, histological slides, or images of body parts may be used without consent as long as all identifying marks that may reveal the patient's identity are removed.
Informed consent for publication should be obtained if there is any doubt. For example, masking the eye region in photographs of participants is not enough to protect the anonymity.
For all research involving human subjects, freely-given, informed consent to participate in the study must be obtained from participants (or their parent or legal guardian in the case of children under 16) and a statement about this should appear in the manuscript.
Consent from the patient may be obtained in a form accepted by the institution or local Ethics Committee and must clearly state how the patient's identifiable information will be used. You can also use the consent form provided on the Sechenov Medical Journal website.
Individuals may consent to participate in a study, but object to having their data published in a journal article. Authors should make sure to receive consent from patients to publish their data prior to submitting their paper to a journal.
The written consent of the patient should be available at the request of the editors during the review of the manuscript, as well as after publication. Manuscripts may be rejected if the required patient consent form cannot be provided.
Ethics statements. Consent statement. The patient consented to the publication of the article “...” in the “Sechenov Medical Journal".





































