Scroll to:
Impact of sprint training on bone health: a literature review of current evidence
https://doi.org/10.47093/2218-7332.2023.14.4.4-16
Abstract
Specific impact of sprint training (ST) on bone health has yet to be fully explored, in particular how it affects bone mineral density (BMD) and bone structure.
Aim. To investigate the ST and bone health relationship between athletes of different training intensities and nonathletes of different ages.
Materials and methods. A search of databases PubMed, Embase, and Pedro was conducted from January 2009 to August 2023. The full texts of all potentially relevant studies were obtained and evaluated by three independent reviewers for inclusion.
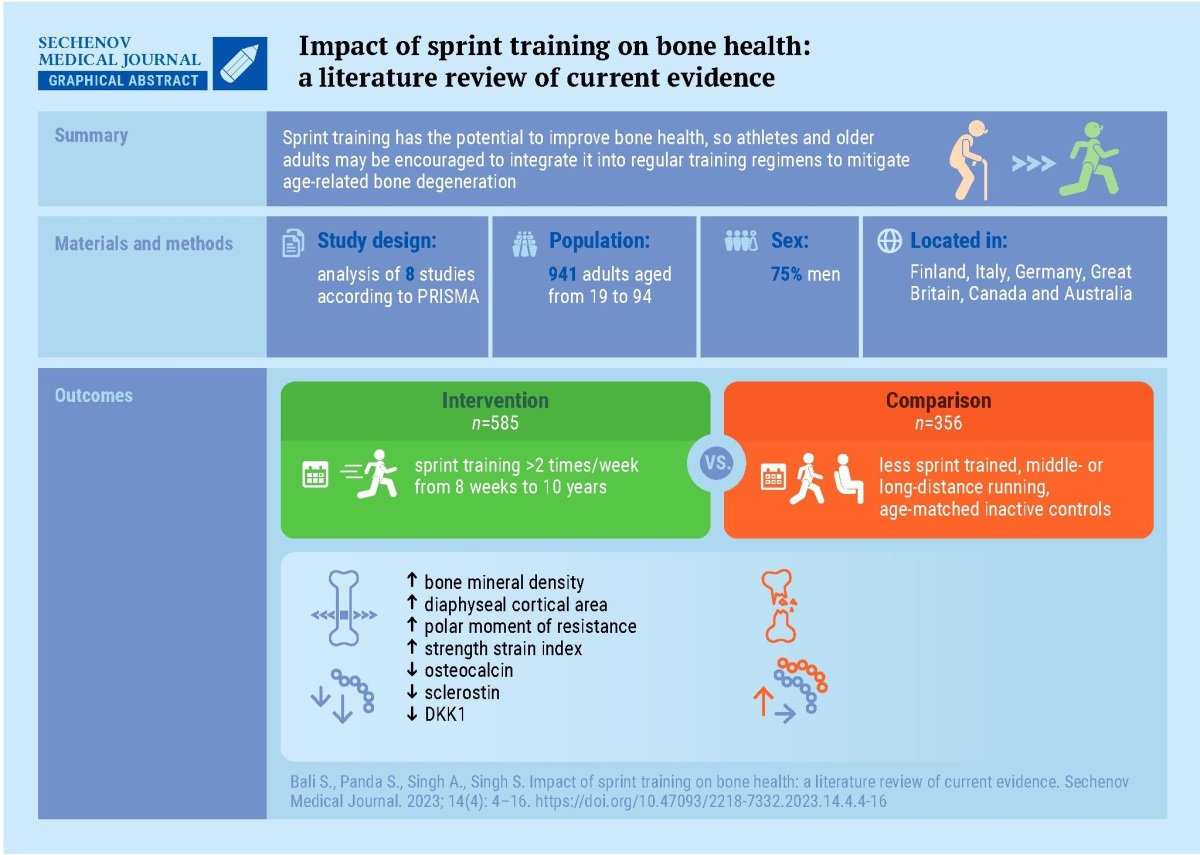
Results. The comprehensive review of eight studies indicates a positive influence of ST on bone health. Sprinters show higher cortical and trabecular BMD in the tibia than controls, with a noted age-related decline in BMD. Short distance runners demonstrate significantly better BMD, counter-movement jump performance, and grip strength compared to long-distance runners. These benefits are consistent across various age groups, including older athletes, with minimal age-related changes in mid-tibial BMD. ST is also associated with a 21% increase in tibial stress-strain index, indicating sustained bone strength, and a reduction in fracture risk in the elderly through downregulation of fracture-related microRNAs.
Conclusion. ST significantly enhances bone health, particularly in improving BMD and bone microarchitecture. Incorporating ST into exercise routines may benefit athletes and older individuals. Further research is essential to understand the mechanisms and develop optimal training protocols for bone health.
Sprint training is crucial in numerous athletic activities, providing a significant competitive benefit in the majority of sports and frequently determining performance success [1][2]. It requires fast muscular energy release to move an athlete forward at the highest possible speed [3]. Sprint training (ST) that combines running with resistance exercises provides effective osteogenic stimulus for maintaining bone density, especially in lower limbs [2][4–11]. According to studies conducted on young and older athletes, sprint and power sports offer an efficient osteogenic stimulus that strengthens bones [1][3][12–14].
Sprint performance has been extensively evaluated using a range of measurements such as peak, mean, and total power, a velocity test or time trial measurement over a set distance [1][15][16]. Depending on the phase an athlete or person is in, ST comprises a variety of interventions and exercise intensities [17]. Compared to aerobic training, sprint interval training (SIT) produces only changes in aerobic fitness and other body composition parameters in premenopausal women [18]. It also helps to improve cardiovascular health in obese people and reduce the inflammatory process in diabetic patients [19][20]. The possible preventative role of ST against osteoporosis and bone health must be investigated in depth to determine the significance of the same in preventing these disorders.
Regular ST positively affects the density and structure of bone, particularly in older and middle-aged athletes, with direction-specific effects on bone health [5][7]. Sprinting has the potential to promote bone formation through the application of mechanical stress and the upregulation of growth factors. Sprinters may have longer forefoot bones due to increased mechanical stress during sprinting, although further research is required to establish any correlation with sprint performance [21].
The effects of repeated-sprint training (RST) on bone health are currently unknown, but high-impact loading from sprinting has been shown to potentially prevent age-related bone loss [6][22]. Sprinters have been found to have elevated bone density in the hips and spine compared to endurance athletes and non-athletic control [11]. Over time, power athletes (such as those who specialize in jumping and sprinting) appear to better maintain bone mass compared to endurance athletes, prominently in men and regardless of any changes in performance. However, the moment of inertia for a cross-sectional area does not seem to show the same difference between male and female athletes [23].
The impact of ST on bone health is attributed to a combination of factors, incorporating factors such as exercise intensity and loading, individual body dimensions, and hormonal attributes [5]. In addition, ST can make beneficial alterations in muscular activity patterns, leading to efficiency improvements through neural pathways and decreased co-contractions [24]. ST can also increase the activity of the enzyme citrate synthase, which is an indicator of muscle oxidative potential [25].
Although the processes underlying the favorable effects of exercise on skeletal health are not yet entirely known, mechanical stimuli, hormones, cytokines, cell signaling pathways and noncoding RNAs are believed to be contributing factors [26].
Bone responds best to exercise throughout adolescence and reduced physical activity during the later stages of life is a contributing factor to the decline in bone mass commonly associated with aging [27, 28]. Regular strength and ST prevent bone deterioration in adults and provides strong osteogenic stimuli to improve bone characteristics at loaded sites. Stimuli such as compression and fluid shear are crucial for osteoblastic activity and the maintenance of a healthy bone mass and density [29]. Sprint-trained athletes exhibit observable structural adaptations as a thickened cortical loaded area, which is one of the reasons for effective direction-specific bending strength [30].
Hence, the objective of this review is to critically evaluate the current evidence on the impact of sprint training on bone health, including the effects on bone mineral density, bone turnover markers, and fracture risk, to provide a comprehensive analysis of the potential benefits and limitations of this training modality for promoting bone health.
MATERIAL AND METHODS
The methodology for conducting this review was based on the PRISMA 2020 checklist [31].
Eligibility criteria
We adhered to the PICOS framework (Population, Intervention, Comparison, Outcomes, and Study) for the design of this study, as described below [32].
Population: Humans of any age, gender, and fitness level who have undergone sprint training.
Intervention: Sprint training is defined as a high-intensity exercise protocol that involves short bursts of maximal effort, with or without additional interventions of any duration or frequency.
Comparison: Any comparison group, including sedentary individuals, those engaging in other types of physical activity, individuals undergoing no intervention, placebo or any forms of exercise.
Outcomes: Bone health, which may be measured using various indicators such as bone mineral density, bone turnover markers or fracture incidence.
Study design: Any experimental and observational research designs, such as randomized controlled trials, non-randomized controlled trials, cohort studies, and case-control studies. However, reviews and conference abstracts were excluded from the study.
Literature search
A systematic literature search was done in electronic databases such as PubMed, Embase, Pedro from January 2009 to August 2023. A variety of keywords like sprinting, exercises, bone health and osteoporosis were used for the same.
The details of the search strategy are provided in Table 1.
Table 1. Search strategy
Таблица 1. Стратегия поиска
|
Search terms / Условия поиска |
Boolean Operator / Логический Оператор |
|
Sprint training, Sprint, High intensity interval training |
OR |
|
Bone and Bones, Bone Density, Bone, Remodeling, Osteoporosis, Bone Regeneration |
OR |
|
Athletes, Sports, Exercise |
OR |
|
English |
AND |
|
2009/01/01–2023/08/31 |
AND |
Study selection
The studies were initially evaluated individually by all four reviewers (SP) (SB) (SS) (AS). All the studies that fulfilled the inclusion or eligibility criteria were included. The difference of opinion was settled by communication among the four regarding the same. In this study, a meta-analysis was not conducted due to the heterogeneity found among the included studies. The study flow chart is depicted in Figure.
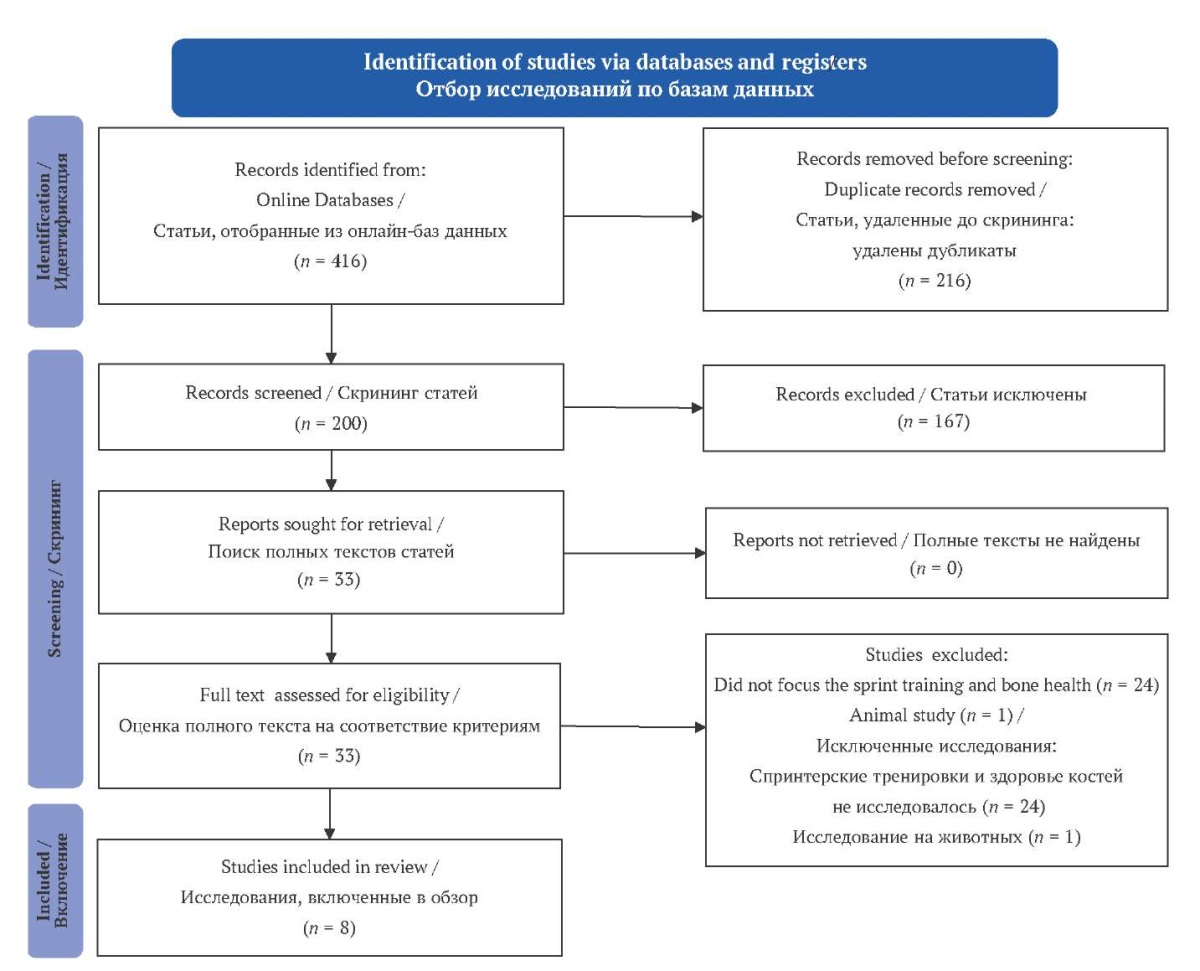
FIG. PRISMA 2020 Flow Diagram.
РИС. Блок-схема PRISMA 2020.
RESULTS
There were 416 studies shortlisted from the databases. After eliminating the duplicates, animal studies and studies that were unrelated to the aim, findings were limited to eight studies. The included studies were carried out in Finland, Italy, Germany, United Kingdom, Canada and Australia. The majority of the research relied on computer tomography to assess the density and other properties of bone.
D.C. Wilks et al. (2009), in a cross-sectional study conducted on sprinters, aimed to see if sprinting enables athletes to maintain bone health till old age. The results of tomographic scans of the lower and upper limbs, as well as trabecular bone mineral density (BMD), indicated that athletes generally had higher cortical and trabecular BMD in the tibia than controls at all stages of life (p < 0.001). However, these outcomes showed a decline with age [8][10].
Another work by U. Gast et al. (2013) studied the physical activity’s impact on the musculoskeletal and neuromuscular performance of adult athletes supported these findings [3]. They used dual X-ray absorptiometry, single-leg hop, and grip tests as outcome measures to examine the sprinters and long-distance runners. Short-distance runners demonstrated significantly greater BMD (p ≤ 0.0012), countermovement jumps and grip force (p ≤ 0.027) compared to long-distance athletes. BMD of athletes was higher than the same-age non-athlete population [3].
Sprinters of different age groups (40–85 years) were examined for bone properties by M.T. Korhonen et al.: bone densitometry and computed tomography values at the distal and mid tibia. BMD at the tibial level was generally greater in athletes than referents (p < 0.05), whereas the other bone characteristics assessed were almost similar between the groups (p > 0.05). At the mid-tibia no age-related changes in total BMD were observed. The findings suggest that middle-aged and older athletes can benefit from ST, as it has a positive impact on bone strength and structure [5].
A randomized control trial examined 72 men (interventional group n = 40, control n = 32) aged between 40–85 years over 20 weeks ST program. BMD measured by computer tomography revealed that the more consistent athletes (adherence >75%) had better strength and bone density (p = 0.007) than the controls included [7]. The results were in accordance with another work which studied effect of ST on 67 athletes aged between 19–84 years. The computed tomographic assessment indicated that sprinters had 21% higher tibial stress-strain index (SSI) (p < 0.001) than the controls. No within-group difference was seen in terms of SSI at the fibula (p = 0.12). Male sprinters appear to maintain tibial bone strength, more than fibular bone strength, as they age [6].
Fracture risk can be assessed by specific systemic markers and molecules derived from bones e.g. micro ribonucleic acids (miRNAs). V. Sansoni et al. [22] in their randomized control study found that ST for 8 weeks downregulates levels of miRNAs related to fracture risk (p < 0.001) and concluded that ST plays an important role in decreasing the risk of fracture in the elderly population.
A longitudinal study of 10 years of follow-up was conducted in Finland, and the researchers assessed the effect of prolonged ST on bone health and aging in 69 male athletes. Densiometric values derived from computer tomography at 10 years of training revealed that well-trained sprinters had better bone properties than less-trained ones (p < 0.05). The main effect was seen in lower and mid-tibial trabeculae [30].
All eight researches concluded that ST has positive benefits on bone health and characteristics. Table 2 summarises the characteristics of the study.
Table 2. Characteristics of included study
Таблица 2. Характеристики включенных исследований
|
# |
Author |
Year |
Sample size & Age |
Study design |
Participants & Intervention |
Outcome measures |
Main findings |
|
1 |
Suominen T.H., et al. [30] |
2021 |
n = 69 (40–85 years) |
Longitudinal |
Male masters athletes. Group 1 – well-trained, (actively competing, sprint training including strength training >2 times/week), n = 36. Group 2 – less-trained (<2 times/week, no strength training, switched to endurance training), n = 33 Regular strength and sprint training for 10 years |
Bone structure, densitometric properties, and strength were measured by pQCT |
Group 1: maintaining or enhancing bone characteristics, Group 2: declining bone characteristics. The most significant disparities were observed in the trabecular vBMD at the distal tibia and the BMC at the mid-tibia’s posterior part |
|
2 |
Sansoni V., et al. [22] |
2018 |
n = 18 (mean age 24) |
Longitudinal |
Healthy, physically active male adults. EXP: repeated-sprint training group, n = 9. CTRL: age-matched inactive controls, n = 9 8 weeks repeated-sprint training, 3 times/week |
Fracture risk-associated miRNA by PCR (RT-qPCR), serum level of DKK1, sclerostin, osteoprotegerin, osteocalcin, osteopontin |
EXP group: after 4 weeks decrease in DKK1 (p < 0.05) and in osteocalcin (p < 0.01); after 8 weeks decrease in sclerostin (p < 0.05) and in expression of circulating miRNA compared to baseline and CTRL |
|
3 |
Suominen T.H., et al. [7] |
2017 |
n = 72 (40–85 years) |
Longitudinal |
Male sprint athletes. EXP: a combination of heavy and explosive strength exercises with sprint training, n = 40. CTRL: usual sprint training schedules, n = 32. A 20 weeks program |
Tibial bone structure and strength |
EXP group: 2.0% increase in ThCO (p = 0.007) compared to CTRL; improvement in total and cortical cross-sectional area ThCO, and area and density-weighted moments of inertia (IminA, IminD) ranged from 1.6–3.2% (p = 0.023–0.006); increase BMC at specific sites and decreased vBMD |
|
4 |
Rantalainen T., et al. [6] |
2014 |
n = 127 (19–39 years & 65–84 years) |
Cross-sectional |
Men. Habitual sprinters, n = 67. Non-athletic, n = 60 |
pQCT to measure bone traits at the mid-shaft of the tibia and fibula |
Sprinters had 21% greater SSI (p < 0.001); 12% larger ToA of the tibia (p < 0.001), 15% larger CoA of the tibia (p < 0.001), 1% lower adjusted cortical density (p = 0.01), compared to non-athletic |
|
5 |
Gast U., et al. [3] |
2013 |
n = 178 (mean age 52.6 – 57.5 years) |
Cross-sectional |
Men athletes and women athletes at the 15th European Masters Championships in Poznań, Poland (2006) Short-distance, n = 50. Middle-distance, n = 19. Long-distance, n = 109 |
aBMD, lean tissue mass, countermovement jump performance, multiple one-leg hopping, and maximal grip force tests |
Older short-distance athletes had significantly higher BMD at key skeletal sites (p < 0.0001 to p < 0.05) and superior neuromuscular performance (p < 0.0001 to p < 0.05) compared to long-distance athletes. Middle-distance athletes showed intermediate results in both BMD and neuromuscular performance compared to the other two groups |
|
6 |
Korhonen M.T., et al. [5] |
2012 |
n = 102 (31–85 years) |
Cross-sectional |
Men. Sprinters, n = 83. Physically active referents, n = 19 |
Bone density, structure, and strength |
Sprinters had significantly higher BMC in the distal and midshaft tibia, with increases ranging from 11% to 48% compared to the reference group. Older sprinters showed reductions in hopping height (ranging from 30.4 ± 4.2 cm in the youngest to 17.0 ± 4.4 cm in the oldest, p < 0.001). Trabecular vBMD of the distal tibia was 12.3% lower in the oldest athletes compared to the youngest (P < 0.05). Strong correlations were found between various bone parameters and factors such as performance in sprint and hop tests, muscle characteristics, and hormone levels, with certain variables like a8verage mechanical power in the braking showing a significant correlation with BMC (p < 0.001) |
|
7 |
Wilks D.C., et al [10] |
2009 |
n = 375 (35–94 years) |
Cross-sectional |
Master athletes in World, European, and British Master Athletics Championships between 2004 and 2006. Sprinters, n = 106. Middle distance runners, n = 52. Long distance runners, n = 93. Race-walkers, n = 49. Sedentary controls, n = 75 |
pQCT measurement of bone mass and geometry |
Male and female sprinters had larger periosteal circumferences in their tibia shafts (4% and 8% respectively, p < 0.001) compared to controls. Sprinters had notably greater trabecular vBMD values (15% for males and 18% for females, p < 0.001) but lower cortical vBMD in the tibia |
|
8 |
Wilks D., et al. [8] |
2009 |
n = 375 (33–94 years) |
Cross-sectional |
Master athletes in World, European, and British Master Athletics Championships between 2004 and 2006. Male master athletes, n = 157. Female master athletes, n = 143. Sedentary controls, n = 75 |
Age-dependency of bone mass and geometry |
In the tibia, athletes consistently exhibited greater diaphyseal CoA, RPol, and trabecular vBMD across all age groups when compared to controls. Among athletes, age was negatively correlated with CoA, RPol (in females), and trabecular vBMD, but this correlation was not observed in the control group (p < 0.01). In contrast, measurements in the radius were similar between athletes and controls across all age groups. When analyzing the combined data, age showed negative correlations with CoA, RPol (in females), cortical vBMD, and trabecular vBMD (in males; p < 0.005) but positive correlations with endocortical circumference (p < 0.001) |
Note: pQCT – Peripheral Quantitative Computed Tomography;
vBMD – Volumetric bone mineral density;
BMC – Bone mineral content; EXP – experimental; CTRL – control;
miRNA – micro ribonucleic acid; PCR – Polymerase chain reaction;
RT-qPCR – Reverse transcription quantitative PCR;
DKK1 – Dickkopf WNT signaling pathway inhibitor 1;
ThCO – mid-tibial cortical thickness; SSI – strength strain index;
ToA – Total Area; CoA – Cortical Area;
aBMD – Areal bone mineral density; RPol – polar moment of resistance.
The Russian version of the table is presented in the supplementary materials to the article on the journal's website.
https://doi.org/10.47093/2218-7332.2023.14.4.4-16-table
DISCUSSION
This systematic review of the literature uncovered a total of eight research papers that focus on investigating the impacts of ST on individuals categorized as healthy, normal-weight adults, as well as athletes. Subjects had better bone characteristics and health than the population who didn’t make ST part of their routine. Furthermore, no side effects were observed in all the subjects that were followed. The link between ST and changes in the bone is clearly of worldwide interest, as evidenced by the research evaluated, which came from different countries of the world, so the focus of the study was to assess the available research and find the effect of ST on bone health. Adults between the age group (45–85) and athletes were included to study effects in the different study populations and age groups. All the studies included found a strong significant link between continuous ST and better bone health. Bone properties and characteristics were better in people who underwent ST, and effects were mainly seen in tibia. Bone health decreased with advancing age, but it was always better than sedentary people at any point of time.
ST has demonstrated beneficial effects on bone health and mechanical loading [6][7][33]. Sprinting efficiency and mechanical characteristics, such as force-velocity-power profile, have shown improvement with the implementation of resisted ST [33]. For male sprinters who are in their mid-to late life research has shown that a twenty-week program involving high-intensity strength and ST can enhance tibial bone structure and strength [7].
Studies have demonstrated that ST can enhance human muscle oxidative potential and boost their capacity for cycling endurance [25]. The appropriate load for ST, according to studies, is the one that decreases an athlete’s velocity by greater than 10% from unloaded sprinting, as it can cause significant alterations in the sprinting method of the athlete [34]. Adding weights to sleds has been employed as a means to enhance sprint acceleration ability, and it has been observed that relatively heavier loads could be more advantageous than lighter loads [35–39]. Overall, ST including resisted ST and heavy sled towing has been shown to positively affect bone health and mechanical loading and might be a practical training strategy for enhancing sprinting performance and bone health.
Body hormones such as estrogen are regulated by physical activity, and the role of these in bone metabolism is a proven fact [40–42]. Physical activity that stimulates estrogen secretion can imitate the effects of hormone replacement therapy and can help osteoporotic menopausal women [43–46]. ST can have a positive impact on bone health by various mechanisms, including hormonal changes. Hormones play a crucial role in bone metabolism, and exercise can affect hormone levels [22]. For instance, a study found that RST for eight weeks increased circulating levels of fracture risk-associated miRNA [22]. A further research study highlighted the positive effects of consistent strength training on the structure and strength of bones in athletes who are middle-aged and above. Furthermore, the investigation highlighted that exercise loading, body size, and hormonal characteristics play a significant role in determining the variations in bone traits among individuals [5]. In addition, a study revealed that the various types of sprint interval sessions could impact the balance of anabolic and catabolic hormones as well as circulating inflammatory cytokines [47]. Therefore, it can be concluded that ST can have beneficial effects on bone health by hormonal changes.
Signaling mechanisms such as Wnt/-catenin, Bone morphogenetic proteins (BMP), osteoprotegerin (OPG)/receptor activator of nuclear factor kappa B ligand (RANKL) govern bone metabolism, and exercise can stimulate numerous signaling pathways to influence osteoblastic and osteoclastic activity [26][48–50]. Noncoding RNAs, including small interfering RNAs (siRNAs), miRNAs, long intervening/intergenic noncoding RNAs (lincRNAs), and circular RNAs (CircRNAs) also have a significant impact on how bone metabolism is regulated by stimulating bone cells [51].
Studies have demonstrated that sprinting can enhance muscle health by activating serine-threonine kinase and promoting protein synthesis and muscle activity, additionally, SIT has been found to increase muscle oxidative potential, cycle endurance capacity and glycogen content in humans [25][51–54]. ST induces metabolic and morphological changes in muscles: it has also been shown to increase muscle Na(+)-K(+)-ATPase concentration, which improves K+ regulation [55], to improve peak performance by 25% [56] and reduce oxidative stress while enhancing antioxidant defense in skeletal muscle and heart [57] According to A. Ross and M. Leveritt (2001), enzyme adaptations are a significant metabolic adaptation to ST, with the enzymes of all three energy systems (phosphate metabolism, glycolysis, aerobic system) exhibiting indicators of training adaptation [17]. Sprint exercise can result in oxidative stress and muscle damage; however, training decreases oxidative stress even more so when exercise is done in severe acute hypoxia [58]. According to D. Morales-Alamo et al. (2014) and M. Esbjörnsson et al. (2009), female individuals exhibit a more pronounced serum growth hormone and insulin response to sprint exercise. This finding may provide a potential explanation for the previously observed phenomenon of greater muscle hypertrophy in women following ST [59][60]. R. Aaserud et al. (1998) conducted a study that revealed that consuming creatine supplements could effectively delay fatigue onset during repeated sprint running bouts [31]. These findings suggest that sprinting can be a valuable exercise for promoting muscle health.
Several studies also that measured bone strength revealed that sprint-trained athletes had greater bone strength than their active counterparts [4][8–10]. Another review by M. Sloth et al. strongly supported the claim that exercise performance, VO2 max, and aerobic capacity increase following sprinting. Our work expands previous findings by demonstrating that not only strength but also bone characteristics are more pronounced in sprinters than in others [53].
The study by M. Sloth et al. on the role of exercise in preventing osteosarcopenia indicated that the best defense against the disease is exercise in conjunction with lifestyle change; however, the exact type, frequency, and intensity of exercise must be explored further [53].
Most of the research included in this review used computer tomography and bone densitometry as an outcome measure to assess bone density and other characteristics, whereas one study used Dual X-ray absorptiometry, one-leg hopping, and maximal grip force tests [3]. Different categories of athletes were also assessed separately, and effects were in favor of ST in all the categories.
In addition to peripheral quantitative computer topographic measures, fasting hormonal measurements of blood concentrations of total testosterone, total estradiol, and sex hormone-binding globulin were also obtained. No substantial Harmonic differences were observed amongst athletes of different ages [5]. Another author, M.C. Rumpf et al. investigated the type of ST and concluded that all types of training (specific, non-specific, and combination) have a good impact on the performance of athletes [61].
The type of ST and their effects separately should be examined in future research to guarantee that the findings can be more precise. Other variations such as body type, past accidents or musculoskeletal issues, lifestyle, life situations, living habits and other stresses might all have an impact on bone health, which were not ruled out in the studies included. Further research with larger sample sizes and specific types of ST is necessary to advance the literature on the variables involved and to comprehensively understand the underlying mechanisms and develop optimal training protocols for maximizing bone health benefits.
The limitation of the study was its focus on descriptive overviews written only in English.
CONCLUSION
Sprint Training (ST) offers notable potential in bolstering bone health, as evidenced by mechanisms such as force stimuli and hormonal effects. The enhancement of BMD, improved bone microarchitecture, and favorable enzyme adaptations underscore the potential efficacy of ST in reducing fracture risks among the elderly. It is paramount to adhere to well-structured training protocols to ensure maximum benefits. The implications of these findings are particularly salient for populations susceptible to osteoporosis. Consequently, integrating ST into regular exercise regimens is recommended for athletes and seniors to mitigate age-associated bone degeneration.
AUTHOR CONTRIBUTION
Seveka Bali and Sougata Panda participated in the development of the article concept, performed the search, analysis, and systematization of literature on the topic of the review and wrote a significant part of the text. Amarjeet Singh and Sonia Singh prepared the introduction and conclusion and finalized the text of the manuscript. Amarjeet Singh developed the general concept of the article andsupervised its writing. All authors approved the final version of the article.
ВКЛАД АВТОРОВ
Севека Бали и Сугата Панда участвовали в разработке концепции статьи, выполнили поиск, анализ и систематизацию литературы по теме обзора и написали значительную часть текста. Амарджит Сингх и Соня Сингх подготовили введение и заключение, а также доработали текст рукописи. Амарджит Сингх разработал общую концепцию статьи и руководил ее написанием. Все авторы одобрили окончательную версию статьи.
Conflict of interests. The authors declare that there is no conflict of interests.
Financial support. The study was not sponsored (own resources).
Конфликт интересов. Авторы заявляют об отсутствии конфликта интересов.
Финансирование. Исследование не имело спонсорской поддержки (собственные ресурсы).
References
1. Faude O., Koch T., Meyer T. Straight sprinting is the most frequent action in goal situations in professional football. J Sports Sci. 2012; 30(7): 625-631. https://doi.org/10.1080/02640414.2012.665940. Epub 2012 Mar 6. PMID: 22394328
2. Nowak A., Straburzynska-Lupa A., Kusy K., et al. Bone mineral density and bone turnover in male masters athletes aged 40-64. Aging Male. 2010 Jun; 13(2): 133-141. https://doi.org/10.3109/13685531003657776. PMID: 20210695
3. Gast U., Belavy D.L., Armbrecht G., et al. Bone density and neuromuscular function in older competitive athletes depend on running distance. Osteoporos Int. 2013 Jul; 24(7): 2033-2042. https://doi.org/10.1007/s00198-012-2234-0. PMID: 23242430
4. Welch J.M., Rosen C.J. Older women track and field athletes have enhanced calcaneal stiffness. Osteoporos Int. 2005 Aug; 16(8): 871-878. https://doi.org/10.1007/S00198-004-1769-0. PMID: 15592922
5. Korhonen M.T., Heinonen A., Siekkinen J., et al. Bone density, structure and strength, and their determinants in aging sprint athletes. Med Sci Sports Exerc. 2012 Dec; 44(12): 2340-2349. https://doi.org/10.1249/MSS.0b013e318267c954. PMID: 22776884
6. Rantalainen T., Duckham R.L., Suominen H., et al. Tibial and fibular mid-shaft bone traits in young and older sprinters and non-athletic men. Calcif Tissue Int. 2014 Aug; 95(2): 132-140. https://doi.org/10.1007/s00223-014-9881-4. PMID: 24925060
7. Suominen T.H., Korhonen M.T., Alen, M., et al. Effects of a 20week high-intensity strength and sprint training program on tibial bone structure and strength in middle-aged and older male sprint athletes: a randomized controlled trial. Osteoporos Int. 2017 Sep; 28(9): 2663-2673. https://doi.org/10.1007/s00198-017-4107-z. PMID: 28623425
8. Wilks D.C., Winwood K., Gilliver S.F., et al. Age-dependency in bone mass and geometry: a pQCT study on male and female master sprinters, middle and long distance runners, race-walkers and sedentary people. J Musculoskelet Neuronal Interact. 2009 OctDec; 9(4): 236-246. PMID: 19949281
9. Wilks D.C., Gilliver S.F., Rittweger J. Forearm and tibial bone measures of distance- and sprint-trained master cyclists. Med Sci Sports Exerc. 2009 Mar; 41(3): 566-573. https://doi.org/10.1249/MSS.0B013E31818A0EC8. PMID: 19204595
10. Wilks D.C., Winwood K., Gilliver S.F., et al. Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: A pQCT study. Bone. 2009 Jul; 45(1): 91-97. https://doi.org/10.1016/j.bone.2009.03.660. PMID: 19332164; PMCID: PMC2832729
11. Piasecki J., McPhee J.S., Hannam K., et al. Hip and spine bone mineral density are greater in master sprinters, but not endurance runners compared with non-athletic controls. Arch Osteoporos. 2018 Jul; 13(1): 72. https://doi.org/10.1007/S11657-018-0486-9. PMID: 29971503; PMCID: PMC6028830
12. Heinonen A., Oja P., Kannus P., et al. Bone mineral density in female athletes representing sports with different loading characteristics of the skeleton. Bone. 1995 Sep; 17(3): 197-203. https://doi.org/10.1016/8756-3282(95)00151-3. PMID: 8541131
13. Heinonen A., Sievanen H., Kyrolainen H., et al. Mineral mass, size, and estimated mechanical strength of triple jumpers' lower limb. Bone. 2001 Sep; 29(3): 279-285. https://doi.org/10.1016/S8756-3282(01)00574-9. PMID: 11557373
14. Bennell K.L., Malcolm S.A., Khan K.M., et al. Bone mass and bone turnover in power athletes, endurance athletes, and controls: a 12-month longitudinal study. Bone. 1997 May; 20(5): 477484. https://doi.org/10.1016/S8756-3282(97)00026-4. PMID: 9145246
15. Rienzi E., Drust B., Reilly T., et al. Investigation of anthropometric and work-rate profiles of elite South American international soccer players. J Sports Med Phys Fitness. 2000 Jun; 40(2): 162169. PMID: 11034438
16. Gomez J.J.H., Marquina V., Gomez Й.ИС On the performance of Usain Bolt in the 100 metre sprint. Eur J Phys. 2013 May; 34(5): 1227-1233. https://doi.org/10.1088/0143-0807/34/5/1227
17. Ross A., Leveritt M. Long-term metabolic and skeletal muscle adaptations to short-sprint training: implications for sprint training and tapering. Sports Med. 2001; 31(15): 1063-1082. https://doi.org/10.2165/00007256-200131150-00003. PMID: 11735686
18. Talanian J.L., Galloway S.D.R., Heigenhauser G.J.F., et al. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol. (1985) 2007 Apr; 102(4): 1439-1447. https://doi.org/10.1152/jap-plphysiol.01098.2006. PMID: 17170203
19. Trilk J.L., Singhal A., Bigelman K.A., Cureton K.J. Effect of sprint interval training on circulatory function during exercise in sedentary, overweight/obese women. Eur J Appl Physiol. 2011 Aug; 111(8): 1591-1597. https://doi.org/10.1007/S00421-010-1777-Z. Epub 2010 Dec 29. PMID: 21190036
20. Zamanpour L., Banitalebi E., Amirhosseini S.E. The effect of sprint training and combined aerobic and strength training on some inflammatory markers and insulin resistance in women with diabetes mellitus (T2dm). Iranian Journal of Diabetes and Metabolism. 2016; 15(5): 300-311.
21. Tanaka T., Suga T., Otsuka M., et al. Relationship between the length of the forefoot bones and performance in male sprinters. Scand J Med Sci Sports. 2017 Dec; 27(12): 1673-1680. https://doi.org/10.1111/sms.12857. Epub 2017 Mar 23. PMID: 28207966
22. Sansoni V., Perego S., Vernillo G., et al. Effects of repeated sprints training on fracture risk-associated miRNA. Oncotarget. 2018 Apr; 9(26): 18029-18040. https://doi.org/10.18632/oncotar-get.24707. PMID: 29719588
23. Ireland A., Mittag U., Degens H. et al. Greater maintenance of bone mineral content in male than female athletes and in sprinting and jumping than endurance athletes: a longitudinal study of bone strength in elite masters athletes. Arch Osteoporos. 2020 Dec; 15(1): 87. https://doi.org/10.1007/S11657-020-00757-W. PMID: 32524289. PMCID: PMC7286845
24. Taylor J., Macpherson T., Spears I., Weston M. The effects of repeated-sprint training on field-based fitness measures: a metaanalysis of controlled and non-controlled trials. Sports Medicine. 2015 Jun; 45(6): 881-891. https://doi.org/10.1007/S40279-015-0324-9. PMID: 25790793
25. Burgomaster K.A., Hughes S.C., Heigenhauser G.J., et al. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. (1985). 2005 Jun; 98(6): 1985-1990. https://doi.org/10.1152/japplphysiol.01095.2004. Epub 2005 Feb 10. PMID: 15705728
26. Tong X., Chen X., Zhang S., et al. The effect of exercise on the prevention of osteoporosis and bone angiogenesis. Biomed Res Int. 2019 Apr; 2019: 8171897. https://doi.org/10.1155/2019/8171897. PMID: 31139653; PMCID: PMC6500645
27. Hannam K., Deere K.C., Hartley A., et al. A novel accelerometer-based method to describe day-to-day exposure to potentially osteogenic vertical impacts in older adults: findings from a multi-cohort study. Osteoporos Int. Mar. 2017; 28(3): 1001-1011. https://doi.org/10.1007/S00198-016-3810-5. Epub 2016 Oct 31. PMID: 27798733; PMCID: PMC5306163
28. Tanaka H., Tarumi T., Rittweger J. Aging and physiological lessons from master athletes. Compr Physiol. 2019 Dec; 10(1): 261296. https://doi.org/10.1002/cphy.c180041. PMID: 31853968
29. Klein-Nulend J., Bacabac R.G., Bakker A.D. Mechanical loading and how it affects bone cells: the role of the osteo-cyte cytoskeleton in maintaining our skeleton. Eur Cell Mater. 2012; 24: 278-291. https://doi.org/10.22203/ecm.v024a20. PMID: 23007912
30. Suominen T.H., Alen M., Tormakangas T., et al. Regular strength and sprint training counteracts bone aging: A 10-year followup in male masters athletes. JBMR Plus. 2021 May; 5(7): e10513. https://doi.org/10.1002/jbm4.10513. PMID: 34258508. PMCID: PMC8260815
31. Aaserud R., Gramvik P., Olsen S.R., Jensen J. Creatine supplementation delays onset of fatigue during repeated bouts of sprint running. Scand J Med Sci Sports. 1998; 8(5, Pt 1): 247-251. https://doi.org/10.1111/j.1600-0838.1998.tb00478.x. PMID: 9809381
32. Amir-Behghadami M., Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020 Jun; 37(6): 387. https://doi.org/10.1136/emermed-2020-209567. Epub 2020 Apr 5. PMID: 32253195
33. Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar; 29; 372: n71. https://doi.org/10.1136/bmj.n71. PMID: 33782057; PMCID: PMC8005924
34. Cross M.R., Lahti J., Brown S.R., et al. Training at maximal power in resisted sprinting: Optimal load determination methodology and pilot results in team sport athletes. PLoS One. 2018 Apr; 13(4): e0195477. https://doi.org/10.1371/journal.pone.0195477. PMID: 29641589; PMCID: PMC5895020
35. Bachero-Mena B., Gonzalez-Badillo J.J. Effects of resisted sprint training on acceleration with three different loads accounting for 5, 12.5, and 20% of body mass. J Strength Cond Res. 2014 Oct; 28(10): 2954-2960. https://doi.org/10.1519/JSC.0000000000000492. PMID: 24736770
36. Bentley I., Sinclair J.K., Atkins S.J., et al. Effect of velocitybased loading on acceleration kinetics and kinematics during sled towing. J Strength Cond Res. 2021 Apr; 35(4): 1030-1038. https://doi.org/10.1519/JSC.0000000000002850. PMID: 30299389
37. Kawamori N., Newton R.U., Hori N., Nosaka K. Effects of weighted sled towing with heavy versus light load on sprint acceleration ability. J Strength Cond Res. 2014 Oct; 28(10): 27382745. https://doi.org/10.1519/JSC.0b013e3182915ed4. PMID: 23539079
38. Cochrane D.J., Monaghan D. Using sprint velocity decrement to enhance acute sprint performance. J Strength Cond Res. 2021 Feb; 35(2): 442-448. https://doi.org/10.1519/JSC.0000000000002707. PMID: 29927891
39. Zisi M., Stavridis I., Agilara G.O., et al. The acute effects of heavy sled towing on acceleration performance and sprint mechanical and kinematic characteristics. Sports. 2022 May; 10 (5): 77. https://doi.org/10.3390/sports10050077. PMID: 35622486; PMCID: PMC9146810
40. Edwards T., Piggott B., Banyard H.G., et al. The effect of a heavy resisted sled-pull mesocycle on sprint performance in junior Australian football players. J Strength Cond Res. 2023 Feb; 37(2): 388-393. https://doi.org/10.1519/JSC.0000000000004269
41. Bemben D.A., Fetters N.L. The independent and additive effects of exercise training and estrogen on bone metabolism. Journal of Strength and Conditioning Research. 2000; 14(1): 114-120.
42. De Souza M.J., West S.L., Jamal S.A., et al. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone. 2008 Jul.; 43(1): 140-148. https://doi.org/10.1016/j.bone.2008.03.013. Epub 2008 Apr 8. PMID: 18486582
43. Xiao C.M., Kang Y., Zhuang Y.C. Effects of elastic resistance band exercise on postural balance, estrogen, bone metabolism index, and muscle strength of perimenopausal period women. J Am Geriatr Soc. 2016 Jun; 64(6): 1368-1370. https://doi.org/10.1111/jgs.14172. PMID: 27321627
44. Gennari L., Merlotti D., Nuti R. Selective estrogen receptor modulator (SERM) for the treatment of osteoporosis in postmenopausal women: focus on lasofoxifene. Clin Interv Aging. 2010 Feb; 5: 19-29. https://doi.org/10.2147/cia.s6083. PMID: 20169039; PMCID: PMC2817938
45. Krum S.A. Direct transcriptional targets of sex steroid hormones in bone. J Cell Biochem. 2011 Feb; 112(2): 401-408. https://doi.org/10.1002/jcb.22970. PMID: 21268060; PMCID: PMC3070194
46. Guerrini M.M., Takayanagi H. The immune system, bone and RANKL. Arch Biochem Biophys. 2014 Nov; 561: 118-123. https://doi.org/10.1016/j.abb.2014.06.003. Epub 2014 Jun 12. PMID: 24929185
47. Gardinier J.D., Mohamed F., Kohn D.H. PTH signaling during exercise contributes to bone adaptation. J Bone Miner Res. 2015 Jun.; 30(6): 1053-1063. https://doi.org/10.1002/jbmr.2432. PMID: 25529455; PMCID: PMC4734644
48. Meckel Y., Nemet D., Bar-Sela S., et al. Hormonal and inflammatory responses to different types of sprint interval training. J Strength Cond Res. 2011 Aug; 25(8): 2161-2169. https://doi.org/10.1519/JSC.0b013e3181dc4571. PMID: 21785293
49. Zhu L., Xu P.C. Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem Biophys Res Commun. 2013 Mar.; 432(4): 612-617. https://doi.org/10.1016/j.bbrc.2013.02.036. Epub 2013 Feb 21. PMID: 23438432
50. Huang Y., Zheng Y., Jia L., Li W. Long noncoding RNA H19 promotes osteoblast differentiation Via TGF-e1/Smad3/HDAC signaling pathway by deriving miR-675. Stem Cells. 2015 Dec; 33(12): 3481-3492. https://doi.org/10.1002/stem.2225. Epub 2015 Oct 23. PMID: 26417995
51. Zuo B., Zhu J., Li J., et al. microRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. J Bone Miner Res. 2015 Feb.; 30(2): 330-345. https://doi.org/10.1002/jbmr.2352. PMID: 25195535
52. Yuan Y., Zhang L., Tong X., et al. Mechanical stress regulates bone metabolism through microRNAs. J Cell Physiol. 2017 Jun.; 232(6): 1239-1245. https://doi.org/10.1002/jcp.25688. Epub 2016 Nov 28. PMID: 27861865
53. Sloth M., Sloth D., Overgaard K., Dalgas U. Effects of sprint interval training on VO2max and aerobic exercise performance: A systematic review and meta-analysis. Scand J Med Sci Sports. 2013 Dec; 23(6): e341-352. https://doi.org/10.1111/sms.12092. Epub 2013 Jul 25. PMID: 23889316
54. Goodman C.A., Hornberger T.A., Robling A.G. Bone and skeletal muscle: Key players in mechanotransduction and potential overlapping mechanisms. Bone. 2015 Nov; 80: 24-36. https://doi.org/10.1016/j.bone.2015.04.014. PMID: 26453495; PMCID: PMC4600534
55. Cariati I., Bonanni R., Onorato F., et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J. Funct. Morphol. Kinesiol. 2021 Jun; 6(2): 55. https://doi.org/10.3390/jfmk6020055. PMID: 34205747; PMCID: PMC8293201
56. McKenna M.J., Schmidt T.A., Hargreaves M., et al. Sprint training increases human skeletal muscle Na+-K-ATPase concentration and improves K+ regulation. J Appl Physiol. 1993 Jul; 75(1): 173-180. https://doi.org/10.1152/jappl.1993.75.1.173. PMID: 8397176
57. Atalay M., Seene T., Hanninen O., et al. Skeletal muscle and heart antioxidant defences in response to sprint training. Acta Physiol Scand. 1996 Oct; 158(2): 129-134. https://doi.org/10.1046/j.1365-201X.1996.540305000.x. PMID: 8899059
58. Linossier M.T., Denis C., Dormois D., et al. Ergometric and metabolic adaptation to a 5-s sprint training programme. Eur J Appl Physiol Occup Physiol. 1993; 67(5): 408-414. https://doi.org/10.1007/BF00376456. PMID: 8299612
59. Morales-Alamo D., Calbet J.A. Free radicals and sprint exercise in humans. Free Radic Res. 2014 Jan; 48(1): 30-42. https://doi.org/10.3109/10715762.2013.825043. Epub 2013 Oct 7. PMID: 23879691
60. Esbjornsson M., Norman B., Suchdev S., et al. Greater growth hormone and insulin response in women than in men during repeated bouts of sprint exercise. Acta Physiologica. 2009 Oct; 197(2): 107-115. https://doi.org/10.1111/J.1748-1716.2009.01994.x. Epub 2009 Apr 27. PMID: 19432586
61. Rumpf M.C., Lockie R.G., Cronin J.B., Jalilvand F. Effect of different sprint training methods on sprint performance over various distances: a brief review. J Strength Cond Res. 2016 Jun; 30(6): 1767-1785. https://doi.org/10.1519/JSC.0000000000001245. PMID: 26492101
About the Authors
S. BaliIndia
Seveka Bali - Physiotherapist, Department of Physical & Rehabilitation Medicine, Post Graduate Institute of Medical Education & Research (PGIMER).
Madhya Marg, Sector 12, Chandigarh, 160012
S. Panda
India
Sougata Panda - Assistant Professor, Department of Physiotherapy, Chandigarh University.
Gharuan, Mohali, Punjab, 140413
Tel.: 08116090083
A. Singh
India
Amarjeet Singh - Professor, Head of the Department of Community Medicine, and School of Public Health, Post Graduate Institute of Medical Education & Research (PGIMER).
Madhya Marg, Sector 12, Chandigarh, 160012
S. Singh
India
Sonia Singh - Assistant Professor, Head of the Department of Physiotherapy, Punjabi University.
Patiala, Punjab, 147002
Supplementary files
|
|
1. Graphic abstract | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(321KB)
|
Indexing metadata ▾ | |

|
2. Table 2. Characteristics of included study | |
| Subject | ||
| Type | Исследовательские инструменты | |
Download
(1005KB)
|
Indexing metadata ▾ | |