Scroll to:
Vascular endothelial growth factor attenuates enhanced spontaneous transdifferentiation of classical and intermediate monocytes in patients with ischemic cardiomyopathy
https://doi.org/10.47093/2218-7332.2025.16.1.20-33
Abstract
Aim. To evaluate the effect of vascular endothelial growth factor A (VEGF-A) on the subpopulation composition of monocytes in the blood mononuclear cell culture of patients with coronary heart disease (CHD), with and without ischemic cardiomyopathy (ICMP).
Materials and methods. A single-center, experimental in vitro study was conducted. The study included 22 patients with CHD: 11 with ICMP, 11 without ICMP, and 10 healthy donors. Blood mononuclei were isolated from venous blood by immunomagnetic separation for CD14 and CD34 antigens, then incubated with and without the addition of VEGF-A 50 ng/mL (control and stimulated samples). After 6 days, the total monocyte content, the proportion of classical CD14++CD16–, intermediate CD14++CD16+, non-classical CD14+CD16++, and transitional CD14+CD16– monocytes were assessed using flow cytofluorimetry.
Results. In groups of patients with CHD and in those groups where the patients were considered relatively healthy, a decrease in the content of CD14++CD16+ in the control and stimulated samples was shown. Only in the CHD group with ICMP relative to the control sample, after VEGF-A stimulation, a statistically significant increase in all CD14+ was found: 10.63% (6.80; 17.64) vs. 15.28% (8.75; 27.99), p < 0.01, and their subpopulations: CD14++CD16−: 6.08% (1.76; 8.84) vs. 8.57% (3.51; 16.8), p < 0.05, CD14++CD16+: 3.64% (2.03; 8.59) vs. 6.26% (3.87; 10.3), p < 0.05. In the same group, a tendency towards an increase in CD14+CD16++ was noted after stimulation: 0.19% (0.18; 1.11) vs. 0.61% (0.37; 1.58), p = 0.062. No differences in the content of all monocytes and their subpopulations after VEGF-A stimulation were found in the CHD without ICMP group nor in the healthy group. The content of CD14+CD16– in all groups in the control and stimulated samples did not differ.
Conclusion. CHD is characterized by a deficiency of all CD14+ cells and intermediate monocytes due to their transdifferentiation. VEGF-A affects the subpopulation composition of monocytes in CHD only in the presence of ICMP by increasing the content of all CD14+ cells, and in their intermediate and classical forms without exceeding the indicators in healthy donors.
Keywords
Cardiovascular diseases remain one of the leading causes of mortality worldwide [1]. Despite significant advancements in conservative and surgical treatments, the search for novel therapeutic strategies for patients with atherosclerosis continues. Among these strategies, the induction of angiogenesis has emerged as a promising approach both for patients with ischaemia and for individuals who have undergone endovascular or open surgical interventions to prevent stent or graft restenosis [2]. Vascular endothelial growth factor A (VEGF-A) has become one of the most extensively studied signalling proteins for stimulating angiogenesis in patients with atherosclerosis. To date, not only recombinant VEGF-A but also a drug for prolonged in vivo synthesis – Neovasculgen® – has been successfully used in the treatment of critical limb ischaemia [3].
VEGF-A is a highly conserved secretory signalling protein that binds to type 1 and type 2 VEGF-tyrosine kinase receptors (vascular endothelial growth factor receptors – VEGFR) on the surface of endothelial cells. VEGFR2 stimulates endothelial cell proliferation, migration, and survival, while VEGFR1 can act as a decoy receptor for VEGF-A [4]. Additionally, VEGF-A can increase vascular permeability, leading to the infiltration of the vascular wall by monocytes, or mediate the development of collateral vessels by recruiting and activating endothelial cells and monocytes [4].
Currently, four immunophenotypically distinct subpopulations of monocytes have been identified in humans: classical, intermediate, non-classical, and transitional cells. The majority of monocytes in circulation are classical CD14++CD16– monocytes, which exhibit pronounced phagocytic and pro-inflammatory properties. These characteristics are also shared by CD14++CD16+ monocytes (intermediate cells). Non-classical CD14+CD16++ monocytes possess limited phagocytic capacity but promote reparative processes by secreting anti-inflammatory cytokines and growth factors [5]. Transitional CD14+CD16– monocytes remain poorly studied; they are likely precursors of classical monocytes or may differentiate from them [6]. It has been demonstrated that the development of coronary heart disease (CHD) and atherosclerosis is associated with an increase in the proportion of intermediate monocytes and a decrease in the number of classical forms. This imbalance is further exacerbated in acute coronary syndrome and myocardial infarction [7][8]. In contrast, in ischaemic cardiomyopathy (ICMP), there is anergy of monocyte differentiation with a deficiency of non-classical forms [9].
Despite existing evidence on the involvement of monocyte subpopulations in the development of ischaemic disorders in atherosclerosis, as well as the practical application of VEGF-A for the treatment of such conditions, the influence of VEGF-A on the differentiation of various monocyte subpopulations possessing angioprotective or pro-inflammatory properties remains unexplored. This effect could have a significant impact on disease progression during VEGF-A therapy.
Aim of the study: To evaluate the influence of VEGF-A on the subpopulation composition of monocytes in peripheral blood mononuclear cell cultures from patients with CHD, depending on the presence of ICMP.
MATERIALS AND METHODS
A single-centre, experimental in vitro study was conducted. Consecutive sampling of patients was carried out from those admitted to the Research Institute of Cardiology – a branch of the Tomsk National Research Medical Centre – between 1 December 2022 and 31 May 2023. The required number of patients in the subgroups was determined during the experimental planning stage using Mead’s resource equation, aiming to achieve a degree of freedom for error equal to 20. During the experiment, the sample size was increased in accordance with Lehr’s formula, based on a pilot analysis of data obtained from studying the initial number of patients determined by Mead’s resource equation.
Patient recruitment
The patient inclusion flowchart is presented in Figure 1. A total of 28 patients were assessed for participation in the study. Exclusion criteria were identified in 6 patients. The study included 22 patients with CHD (19 men and 3 women) aged between 54 and 70 years, of whom 11 had ICMP and 11 did not. During the first 4 months, 11 CHD patients with ICMP and 8 patients with ICMP were recruited. Over the following 2 months, only patients with ICMP were included in the study to increase the sample size to the calculated value (n = 11).
Inclusion criteria:
- Age: 18 to 70 years;
- Signed informed consent to participate in the study;
- History of myocardial infarction more than 6 months prior;
- Additional criterion for the ICMP group: left ventricular systolic dysfunction (ejection fraction less than 40%), accompanied by one or more of the following:
− Myocardial infarction or myocardial revascularisation at least 6 months prior;
− Stenosis of the left main coronary artery > 75%;
− Stenosis of two or more coronary arteries > 75% [10].
Exclusion criteria:
- Acute infectious diseases within 3 weeks prior to the study (n= 2);
- Autoimmune or allergic diseases in the acute phase (n= 2);
- Malignant neoplasms (n= 1);
- Viral hepatitis (n= 1);
- Syphilis (n = 0);
- Human immunodeficiency virus (HIV) infection (n = 0);
- Erythropoiesis-stimulating (n= 1) or immunosuppressive therapy (n = 1) within 3 weeks prior to the study.
The control group consisted of 10 generally healthy donors (7 men and 3 women, median age 57.5 [ 48.0; 65.5] years) without any cardiovascular diseases or related complaints, who provided informed consent to participate in the study (Fig. 1).
Isolation of blood mononuclear cells
The study material consisted of 30 ml of blood collected in a single draw from the cubital vein in the morning on an empty stomach, prior to physical activity and diagnostic or therapeutic procedures. The blood was stabilised with heparin (25 IU/ml).
Blood mononuclear cells were isolated using density gradient centrifugation with Ficoll (density 1.077 g/cm³) (LLC NPO “PanEco”, Russian Federation). After washing the mononuclear cells twice with 0.5% PBS (pH = 7.2), immunomagnetic separation was performed using CD14 MicroBeads and CD34 MicroBead Kit (“Miltenyi Biotec B.V. & Co. KG”, Germany), MS separation columns (“Miltenyi Biotec B.V. & Co. KG”, Germany), and a MiniMACS magnet (“Miltenyi Biotec B.V. & Co. KG”, Germany) according to the manufacturer’s instructions. The proportion of CD14+ cells (all monocytes) and CD34+ cells (stem and progenitor haematopoietic cells, as potential precursors of monocytes, also present in the blood) in the culture was 80-85% and 3–5%, respectively.
Cell viability was assessed using a 0.1% trypan blue test (LLC NPO “PanEco”, Russian Federation). Cells with a viability of at least 96% were seeded into 2 wells of a 24-well plate at 106 cells per well. The cells were incubated for 6 days under 5% CO2 in complete culture medium (RPMI-1640 medium (LLC NPO “PanEco”, Russian Federation), foetal bovine serum, L-glutamine, penicillin-streptomycin) with the addition of 50 ng/ml recombinant human VEGF-A (“Cloud-Clone Corp.”, USA) to one of the wells. After 3 days of incubation, a partial medium change was performed, and the stimulant was re-added at the same dose. The sample with recombinant VEGF-A was considered stimulated, while the sample without VEGF-A served as control. After 6 days, the cells were detached from the plate surface by incubation with 500 µl of 0.05% trypsin-EDTA solution (LLC NPO “PanEco”, Russian Federation) per well for 5 minutes at 37 °C. After washing the cells with 500 µl of 0.5% PBS, the pellet was resuspended, and the cells were used for flow cytometry (Fig. 2).
Immunophenotyping of monocyte subpopulations
Fluorescence intensity was measured using a “CytoFLEX” flow cytometer (“Beckman Coulter International S.A.”, USA) with the “CytExpert 2.3” software application (“Beckman Coulter International S.A.”, USA). The boundaries for positive fluorescence signals were established using FMO (Fluorescence Minus One) controls, as a third antibody (not presented in this publication) was also used in the study. However, the determinants CD14 and CD16 were evaluated independently of the expression of its ligand. The proportion of cells positive for each marker was assessed as a percentage of the total number of events, excluding the region of small objects (FSC less than 100×104).
Statistical data analysis
For the presentation of results, the median and interquartile range (25th and 75th percentiles) were calculated. The normality of the distribution in samples was assessed using the Kolmogorov-Smirnov test. Given the deviation of the sample data from a normal distribution, comparative analysis was performed using Mann-Whitney test (for independent samples) and Wilcoxon test (for dependent samples), with Benjamini-Hochberg correction for multiple comparisons. Statistical significance of differences in relative indicators was evaluated using Pearson’s chi-square test. Spearman’s correlation coefficients were calculated. The strength of association was assessed using Chaddock’s scale: a correlation coefficient of 0–0.3 was considered very weak, 0.3–0.5 as weak, 0.5–0.7 as moderate, 0.7–0.9 as strong, and 0.9–1 as very strong. The results of the statistical analysis were considered significant at a level of p < 0.05. Statistical data analysis was performed using “Statistica 10.0” software (StatSoft Inc., USA).
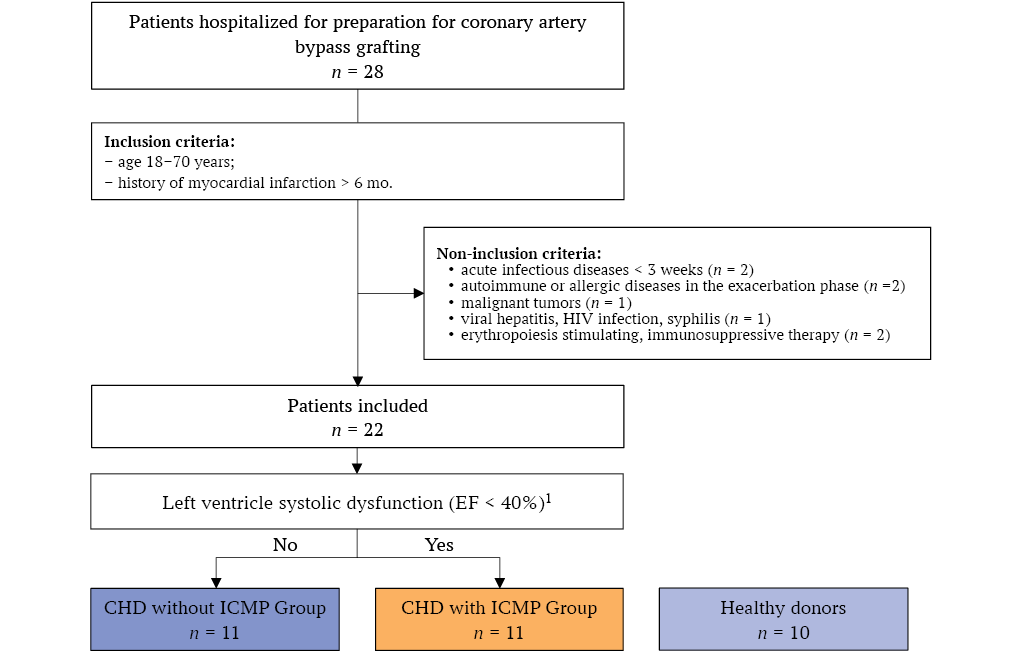
FIG. 1. Study flowchart.
Note: HIV – human immunodeficiency virus; CHD – coronary heart disease; ICMP – ischemic cardiomyopathy; EF – ejection fraction.
1 With ≥ 1 feature: myocardial revascularization > 6 mo.; left main coronary artery stenosis > 75%; stenosis of two or more coronary arteries > 75%.
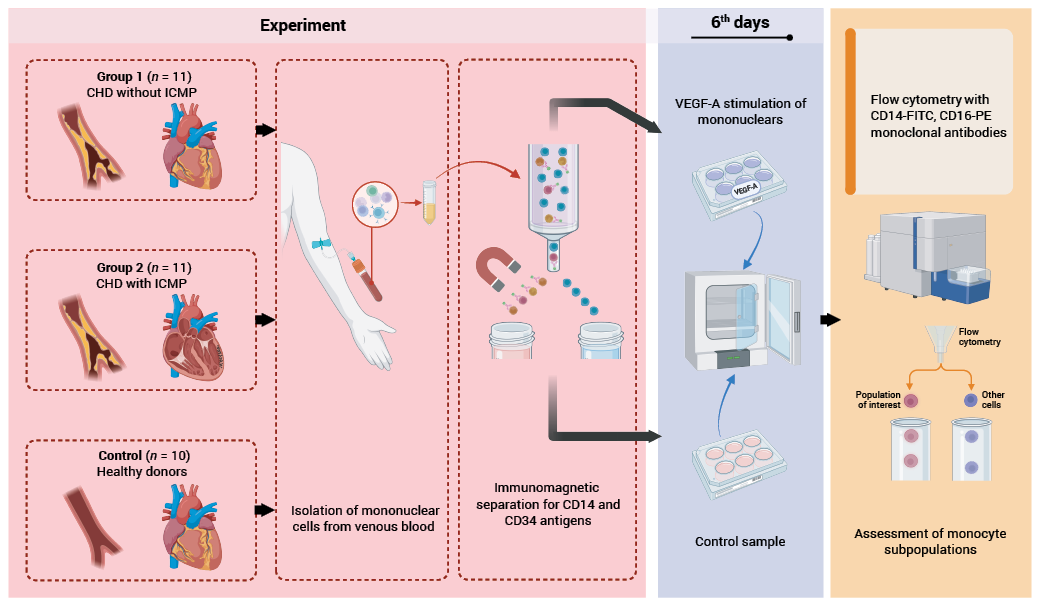
FIG. 2. Experimental design.
Note: CHD – coronary heart disease; ICMP – ischemic cardiomyopathy; VEGF-A – vascular endothelial growth factor A.
RESULTS
Baseline Patient Characteristics
Patients with CHD showed no significant differences in age, sex, or prescribed therapy between the studied groups (with and without ICMP) (Table 1). Calcium channel blockers were not administered to patients with ICMP due to their negative inotropic effects. The majority of patients in both groups presented with angina pectoris of functional class II-III and heart failure of functional class II-III, as classified by the New York Heart Association (NYHA).
The Effect of VEGF-A on Monocyte Subpopulation Composition
The addition of VEGF-A to the mononuclear cell culture of healthy donors did not alter either the total monocyte count or the ratio of their subpopulations. Notably, the total monocyte count in the culture remained at approximately 40%, irrespective of the presence of the stimulant (Table 2).
In samples from patients without ICMP, a threefold reduction in the proportion of intermediate CD14++CD16+ monocytes was observed compared to the healthy donor group, while the percentages of other monocyte immunophenotypes and the total monocyte count remained comparable. The addition of VEGF-A to the mononuclear cell culture in these patients, as in healthy donors, had no effect on the total monocyte count or their subpopulation composition (Table 2).
In patients with ICMP, similar to those without ICMP, a deficiency in the number of intermediate CD14++CD16+ monocytes was observed in the mononuclear cell culture, regardless of the presence of the stimulant. In unstimulated samples from patients with ICMP, a profound deficit in the total monocyte count (fourfold) and intermediate CD14++CD16+ monocytes (tenfold) was identified compared to the healthy donor group. These two parameters showed a statistically significant increase under the influence of VEGF-A relative to the control sample, although they did not reach the levels observed in healthy donors. Unlike in patients without ICMP, the proportion of classical CD14++CD16− monocytes in patients with ICMP significantly increased after incubation with VEGF-A compared to the unstimulated sample, reaching levels comparable to those in the healthy donor group (Table 2).
No statistically significant differences in the number of non-classical CD14+CD16++ monocytes and transitional CD14+CD16− monocytes were observed between the control and VEGF-A-stimulated samples across the studied groups. However, only in patients with ICMP was there a notable trend (p = 0.062) towards an increase in the proportion of non-classical CD14+CD16++ monocytes in the cell culture containing VEGF-A compared to the unstimulated sample (Table 2).
Correlation of Monocyte Subpopulations with Total Monocyte Count
(1) Among All Patients with Coronary Heart Disease
Correlation analysis conducted among all patients with CHD (both with and without ICMP) revealed a strong positive association between the total monocyte count and the proportion of intermediate CD14++CD16+ and classical CD14++CD16− monocytes in the control sample (Fig. 3A). The strength of this association remained unchanged following the addition of VEGF-A to the culture (Fig. 3B). A similarly positive, moderate-strength correlation between the total monocyte count and the percentage of transitional CD14+CD16− was observed in the control sample; this correlation strengthened to very strong in the presence of VEGF-A. Additionally, a moderate-strength association between the total monocyte count and the number of non-classical CD14+CD16++ monocytes, identified in the unstimulated mononuclear cell culture, weakened to a weak correlation under the influence of VEGF-A (Fig. 3C, 3D).
(2) Depending on the Presence of Ischemic Cardiomyopathy and VEGF-A Stimulation
The analysis of correlations between the content of individual monocyte subpopulations and total monocyte count revealed associations of variable strength, depending both on the presence of ICMP and the addition of VEGF-A to the mononuclear cell culture (Fig. 4).
A common feature found in both groups of patients and in healthy donors was a strong positive correlation between the total monocyte count and the proportion of intermediate monocytes in the control (across all categories of individuals) and VEGF-A-stimulated sample (in the control group and in patients without ICMP). A distinctive characteristic of patients with ICMP was a strong positive correlation between the total monocyte count and the percentages of three monocyte subpopulations (classical, intermediate, and transitional) in the absence of the stimulant. However, upon the addition of VEGF-A, the correlation with the number of intermediate monocytes disappeared. Contrastingly, in patients without ICMP, a strong positive correlation of the total monocyte count with the proportion of classical and intermediate cells identified in the unstimulated sample was, after stimulation with VEGF-A, accompanied by an additional strong positive correlation with the level of transitional monocytes (Fig. 4).
Table 1. Baseline characteristics of patients with coronary heart disease in the studied groups
|
Feature |
Coronary heart disease |
p value |
|
|
without ICMР (n = 11) |
with ICMР (n = 11) |
||
|
Men, n (%) |
10 (91) |
11 (100) |
n.s. |
|
Women, n (%) |
1 (9) |
- |
n.s. |
|
Age, years |
63,5 (58,0; 67,5) |
60,5 (56,5; 64,0) |
n.s. |
|
Stable angina: |
|||
|
Class II, n (%) |
2 (18) |
3 (27) |
n.s. |
|
Class III, n (%) |
8 (73) |
7 (64) |
n.s. |
|
Class IV, n (%) |
1 (9) |
1 (9) |
n.s. |
|
Left ventricular ejection fraction, % |
59,25 (50,00; 67,50) |
30,50 (22,75; 36,50) |
< 0,001 |
|
NYHA classification of heart failure |
|||
|
Class I, n (%) |
2 (18) |
1 (9) |
n.s. |
|
Class II, n (%) |
4 (36) |
7 (64)? |
n.s. |
|
Class III, n (%) |
5 (46) |
3 (27) |
n.s. |
|
Medications: |
|||
|
Long-acting nitrates, n (%) |
7 (64) |
6 (55) |
n.s. |
|
β1 blockers, n (%) |
10 (91) |
9 (82) |
n.s. |
|
Calcium channel blockers, n (%) |
7 (64) |
0 |
0,001 |
|
ACE inhibitors, n (%) |
3 (27) |
5 (46) |
n.s. |
|
Antiplatelet agents, n (%) |
8 (73) |
9 (82) |
n.s. |
|
Statins, n (%) |
9 (82) |
10 (91) |
n.s. |
Note: ACE – angiotensin-converting enzyme; ICMР – ischemic cardiomyopathy; n.s. – not significant, не значимо; NYHA – New York heart association.
Table 2. Content of monocyte subpopulations in the mononuclear cell culture in the control and in the VEGF-A stimulated samples in all groups studied
|
Monocyte content |
Coronary heart disease |
Healthy donors (n = 10) |
|||||||
|
without ICMP (n = 11) |
with ICMP (n = 11) |
||||||||
|
Control |
VEGF-A |
p value |
Control |
VEGF-A |
p value |
Control |
VEGF-A |
p value |
|
|
All monocytes CD14+/++, % |
17,79 (7,15; 35,63) |
21,50 (7,15; 38,8) |
n.s. |
10,63 (6,80; 17,64)b |
15,28 (8,75; 27,99)а |
<0,01 |
40,42 (21,70; 47,62) |
41,25 (20,55; 46,69) |
n.s. |
|
Classical monocytes CD14++CD16−, % |
5,45 (2,13; 15,27) |
8,45 (3,23; 9,09) |
n.s. |
6,08 (1,76; 8,84) |
8,57 (3,51; 16,8) |
<0,05 |
10,72 (6,73; 2,04) |
10,66 (6,37; 12,31) |
n.s. |
|
Intermediate monocytes CD14++CD16+, % |
9,12 (5,23; 23,06)a |
11,10 (4,60; 23,9)a |
n.s. |
3,64 (2,03; 8,59)b |
6,26 (3,87; 10,3)а |
<0,05 |
30,42 (13,36; 35,77) |
34,81 (13,73; 40,85) |
n.s. |
|
Non-classical monocytes CD14+CD16++, % |
0,86 (0,47; 1,28) |
1,06 (0,22; 1,81) |
n.s. |
0,19 (0,18; 1,11) |
0,61 (0,37; 1,58) |
0,062 |
0,92 (0,56; 1,27) |
0,89 (0,33; 1,45) |
n.s. |
|
Transitional monocytes CD14+CD16−, % |
2,90 (1,49; 4,47) |
2,23 (1,58; 4,59) |
n.s. |
2,48 (1,53; 4,80) |
2,40 (1,70; 3,51) |
n.s. |
2,53 (2,11; 4,78) |
2,49 (1,92; 6,204) |
n.s. |
Note: ICMP – ischemic cardiomyopathy; VEGF-A – vascular endothelial growth factor A; n.s. – not significant;
a – p < 0.05 in comparison to the same sample in healthy donors; b – p < 0.01 in comparison to the same sample in healthy donors.
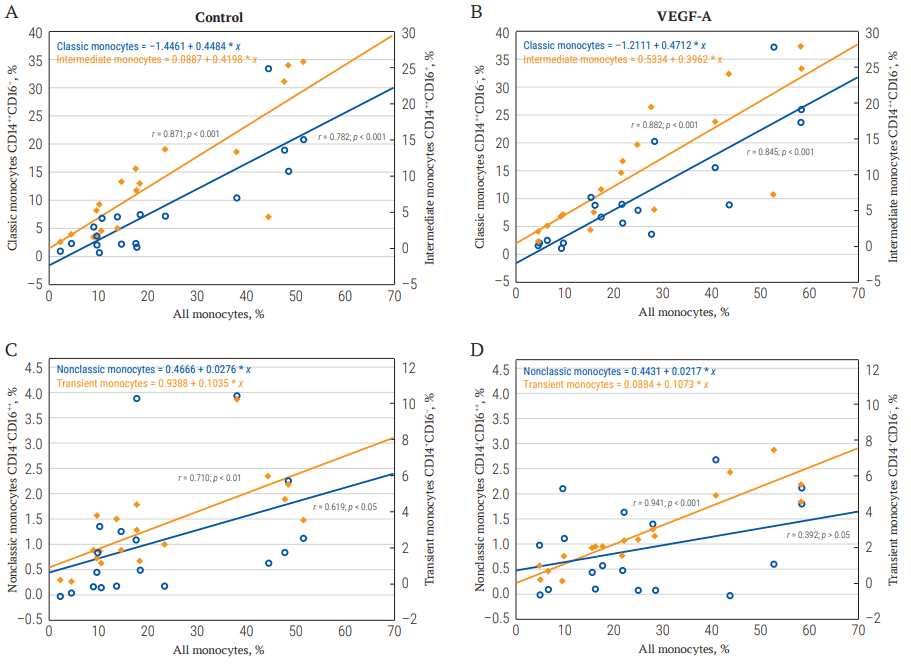
FIG. 3. The correlation of the total number of monocytes in culture with their individual subpopulations in the control and in the VEGF-A stimulated samples in all patients with coronary heart disease.
Note: VEGF-A – vascular endothelial growth factor A.
- The content of classical CD14++CD16−and intermediate CD14++CD16+ monocytes in the control sample.
- The content of classical CD14++CD16−and intermediate CD14++CD16+ monocytes after VEGF-A stimulation.
- The content of non-classical CD14+CD16++ and transitional CD14+CD16−monocytes in the control sample.
- The content of non–classical CD14+CD16++ and transitional CD14+CD16−monocytes after VEGF-A stimulation.
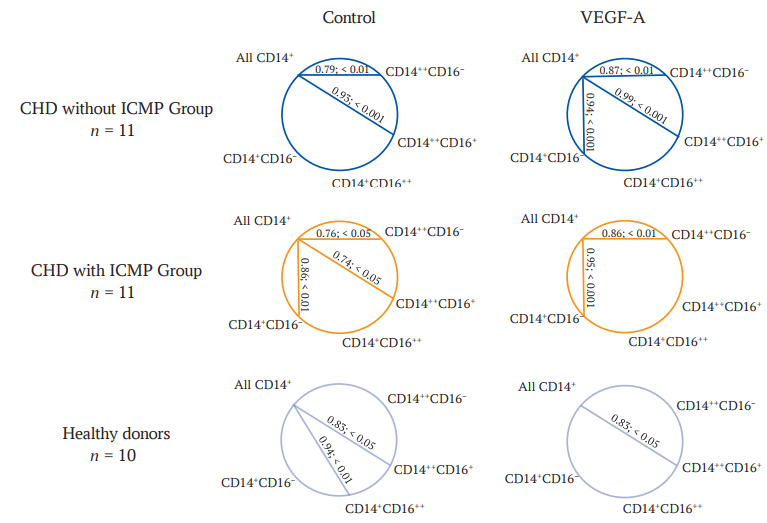
FIG. 4. The correlation of the total number of monocytes in culture with their individual subpopulations in the control and in the VEGF-A stimulated samples in all groups studied.
Note: VEGF-A – vascular endothelial growth factor A; CHD – coronary heart disease; ICMP – ischemic cardiomyopathy; CD14++CD16– – classic monocytes; CD14++CD16+ – intermediate monocytes; CD14+CD16++ – non-classic monocytes; CD14+CD16– – transient monocytes.
DISCUSSION
The study revealed a consistent trend across all three groups of examined individuals – CHD patients without ICMP, patients with ICMP, and healthy donors – characterised by a low proportion of CD14+ cells after culturing compared to their initial purity (80-85%) after isolation, regardless of VEGF-A stimulation. In the samples of healthy donors, approximately 40% of CD14+ cells remained after 6 days of culturing, whereas in ICMP patients, this proportion dropped to 10–15%, despite maintaining cell viability at ≥96% (indicating that the cells remained alive but had lost their monocytic identity). This phenomenon can be attributed to the high plasticity of monocytes, which enables them to differentiate into various subpopulations, as well as into macrophages [11], and even fibrocytes and fibroblasts, which lack the CD14 surface marker [12]. Additionally, a small subset of monocytes, specifically CD14+CD34+VEGFR2+ endothelial progenitor cells (comprising 1–6% of blood monocytes), can directly differentiate into endotheliocytes when exposed to an endothelial (pro-angiogenic) microenvironment [13, 14].
Notably, in healthy donors, monocytes also undergo spontaneous transdifferentiation during culturing (only 40% of CD14+ cells remain). In CHD patients without ICMP, this process tends to be more pronounced (approximately 18% CD14+ cells remain), while in patients with ICMP, it is enhanced considerably, reaching statistical significance (only 10% CD14+ cells remain). The latter may represent an in vivo pathogenetic mechanism contributing to the development of ICMP, which is characterized by diffuse myocardium fibrosis [15], likely driven by the excessive transdifferentiation of monocytes into fibroblasts and fibrocytes.
Analysis of the subpopulation composition of monocytes in native mononuclear cell cultures revealed distinct differences in the content of specific monocyte subsets between the two CHD patient groups. In CHD patients without ICMP, the trend towards a reduction in the total CD14+ cells was accompanied by a significant decrease in intermediate CD14++CD16+ monocytes. In contrast, ICMP patients exhibited a profound deficit of intermediate CD14++CD16+ monocytes and a marked tendency towards a deficiency of non-classical CD14+CD16++ monocytes, alongside a statistically significant reduction in total CD14+ cells. Intermediate and non-classical monocytes represent activated monocytic forms, and their proportion increases in various disease states [16]. This may explain why their numbers declined most significantly during culturing in both patient groups, influencing the overall monocyte count. Such interpretation is supported by a positive correlation between the total monocyte count and the proportion of intermediate CD14++CD16+ monocytes in both patient groups, whereas a correlation with non-classical CD14+CD16++ monocytes was observed only in healthy donors. Furthermore, intermediate monocytes are known to produce the highest levels of reactive oxygen species under unstimulated conditions [16], and an elevated count of these cells is linked to an increased risk of cardiovascular diseases [7–9][17].
The addition of VEGF-A to mononuclear cell cultures from ICMP patients increased both the total monocyte count and the proportion of intermediate CD14++CD16+ and classical CD14++CD16− monocytes compared to control sample. In contrast, VEGF-A stimulation had no effect on the studied parameters in CHD patients without ICMP or in healthy donors. Given that the primary cause of the reduction in CD14+ monocytes during culturing appears to be their transdifferentiation into CD14− cells (fibrocytes, fibroblasts, and endothelial cells), it is plausible that VEGF-A inhibits this process in ICMP patients, thereby preserving a greater number of intermediate and classical monocytes.
Classical CD14++CD16− monocytes, which participate in innate immune responses following extravasation [11][18], demonstrate the highest level of plasticity [11]. Consequently, their level should decrease the most during culturing as they differentiate into CD14− cells. However, according to our observations, patients with CHD showed the greatest deficit of intermediate monocytes, while the reduction in classical monocytes was less pronounced. This data indicates that intermediate monocytes exhibit more active transdifferentiation during the culturing process. The comparable number of classical CD14++CD16− monocytes in CHD patients and healthy donors is consistent with their high plasticity, as they represent the predominant population of blood monocytes [9], whereas intermediate CD14++CD16+ monocytes are the most abundant population in culture. This suggests that, under culturing conditions, classical monocytes initially differentiate into intermediate forms, as observed in vivo [19], and subsequently undergo transdifferentiation into non-monocytic cells. The more pronounced reduction in intermediate monocytes compared to classical monocytes is likely due to the fact that the majority of classical monocytes have already transitioned into intermediate forms after 6 days of culturing. Surprisingly, VEGF-A inhibited the differentiation and transdifferentiation of monocytes in patient-derived cultures, despite its primary association with adaptation to hypoxia and angiogenesis rather than to monocytopoiesis and inflammation. This effect can be attributed to the expression of pro-inflammatory VEGFR1 and pro-angiogenic VEGFR2, which are present in 5-10% of monocytes [20]. Notably, the corrective influence of VEGF-A was observed exclusively in patients with ICMP and was absent in CHD patients without ICMP and in healthy donors. This phenomenon may stem from receptor hyperexpression or enhanced intracellular signaling through the ‘cytochrome P450 4A/F – 20-hydroxyeicosatetraenoic acid’ pathway, which is upregulated by hypoxia [21]; in ICMP, this hypoxia becomes chronic due to widespread myocardial ischemia [14].
Studies have demonstrated that the elimination of VEGF results in mitochondrial fragmentation, suppression of cellular metabolism, and death of autophagic cells [21]. These effects are mediated by the transcription factor FOXO (forkhead box protein), which activates autophagy and promotes the survival of hematopoietic stem cells under metabolic stress. In endotheliocyte cultures, VEGF exposure deactivates FOXO1 and inhibits cell death [22]. While the correlation patterns for classical and intermediate monocytes did not significantly differ in the presence or absence of VEGF-A, the proportion of non-classical and transitional monocytes in the absence of VEGF-A stimulation correlated with the total monocyte count. However, upon the addition of VEGF-A, the correlation between the total monocyte count and transitional monocytes strengthened, whereas the correlation with non-classical monocytes was lost. Furthermore, a notable trend towards a 3-fold increase (p = 0.062) in the proportion of non-classical monocytes in cultures from ICMP patients in the presence of VEGF-A underscores VEGF-A’s ability to attenuate transdifferentiation and address the deficiency of non-classical monocytes. This effect is advantageous, as non-classical monocytes exhibit protective properties toward the endothelium by removing immune complexes and dead cells from its surface; it is the deficiency of these cells in the blood that is characteristic for patients with ICMP [9].
The observed differences in correlation patterns among the three groups of individuals, depending on the presence or absence of VEGF-A in cultures, indicate that CHD is associated with qualitative impairments in monocyte reactivity, which are most pronounced in ICMP. These disturbances are characterized by a dysregulation of the spontaneous transdifferentiation of classical, non-classical, and transitional monocytes in response to both in vitro conditions and VEGF-A stimulation. The ability of intermediate monocytes to undergo spontaneous transdifferentiation is heightened in CHD, irrespective of the presence of ICMP. However, only in ICMP is this phenomenon accompanied by a reduction in the total monocyte count in culture and is mitigated by the presence of VEGF-A.
Limitations of the Study and Directions for Future Research
The findings are applicable to patients from the West Siberian region who have a history of myocardial infarction dating back at least 6 months and who have signs of multivessel coronary artery disease. The data indicating that VEGF-A normalises the subpopulation composition of monocytes suggest that VEGF-A could be utilised for CHD therapy to promote angiogenesis without the risk of exacerbating atherosclerosis. Additionally, insights into the pathologically enhanced transdifferentiation of monocytes in CHD could inform the development of novel therapeutic strategies aimed at regulating this transition.
CONCLUSION
The development of CHD, irrespective of the presence of ICMP, is associated with a reduction in the number of intermediate monocytes in vitro due to their spontaneous transdifferentiation. This phenomenon is most pronounced in ICMP and represents a pathogenetic factor of this condition. Stimulation of blood mononuclear cells from CHD patients with the cytokine VEGF-A in vitro modulates the subpopulation composition of monocytes exclusively in individuals with ICMP, mitigating the enhanced transdifferentiation and preventing excessive loss of cells with classical, intermediate, and, to some extent, non-classical immunophenotypes, which demonstrates a protective effect of VEGF-A. At the same time, VEGF-A does not promote excessive accumulation of cells with pro-inflammatory properties (intermediate and classical forms). In CHD without ICMP, VEGF-A has no impact on the subpopulation composition of monocytes, suggesting a potential therapeutic opportunity for the use of this cytokine in CHD treatment without the risk of exacerbating atherogenesis.
Ethics statements. The studies were conducted in accordance with the ethical principles set out in the Helsinki Declaration (1975) and with the permission of the local ethics committee of the Siberian State Medical University of the Ministry of Health of the Russian Federation (protocol No. 9299 dated November 28, 2022). Informed consent to participate in the study was obtained from all examined individuals.
Data availability. The data confirming the findings of this study are available from the authors upon reasonable request. Data and statistical methods used in the article were examined by a professional biostatistician on the Sechenov Medical Journal editorial staff.
Conflict of interest. The authors declare that there is no conflict of interests.
Financing. The study was supported by the Russian Science Foundation grant No. 22-25-20038, https://rscf.ru/project/22-25-20038/ and funds from the Administration of the Tomsk Region.
Соответствие принципам этики. Исследования проводились в соответствии с этическими принципами, изложенными в Хельсинкской декларации (1975), и с разрешения локального этического комитета ФГБОУ ВО СибГМУ Минздрава России (протокол №9299 от 28.11.2022). У всех обследованных лиц было получено информированное согласие на участие в исследовании.
Доступ к данным исследования. Данные, подтверждающие выводы этого исследования, можно получить у авторов по обоснованному запросу. Данные и статистические методы, представленные в статье, прошли статистическое рецензирование редактором журнала – сертифицированным специалистом по биостатистике.
Конфликт интересов. Авторы заявляют об отсутствии конфликта интересов.
Финансирование. Исследование выполнено за счет гранта Российского научного фонда № 22-25-20038, https://rscf.ru/project/22-25-20038/ и средств администрации Томской области.
References
1. Kim S.J., Mesquita F.C.P., Hochman-Mendez C. New Biomarkers for Cardiovascular Disease. Tex Heart Inst J. 2023 Oct 16; 50(5): e238178. https://doi.org/10.14503/thij-23-8178. PMID: 37846107
2. Kastora S.L., Eley J., Gannon M., et al. What Went Wrong with VEGF-A in Peripheral Arterial Disease? A Systematic Review and Biological Insights on Future Therapeutics. J Vasc Res. 2022; 59(6): 381–393. https://doi.org/10.1159/000527079. Epub 2022 Nov 15. PMID: 36380643
3. Mikhaylichenko V.Yu., Tsaturyan A.B., Khizriev S.M., et al. Experience in the use of therapeutic angiogenesis with neovasculgen in patients with non-shuntable lesion of the arteries of the lower extremities. Tavricheskiy mediko-biologichesky vestnik. 2022; 25(2): 55–60 (In Russian). EDN: FFLYZT
4. Wiszniak S., Schwarz Q. Exploring the Intracrine Functions of VEGF-A. Biomolecules. 2021 Jan 19; 11(1): 128. https://doi.org/10.3390/biom11010128. PMID: 33478167
5. Zhang H., Wang S.L., Sun T., et al. Role of circulating CD14++CD16+ monocytes and VEGF-B186 in formation of collateral circulation in patients with hyperacute AMI. Heliyon. 2023 Jun 29; 9(7): e17692. https://doi.org/10.1016/j.heliyon.2023.e17692. PMID: 37456037
6. Vins M.V., Chumakova S.P., Urazova O.I., et al. Monocyte subpopulations of blood and bone marrow in patients with chronic heart failure. Bulletin of Siberian Medicine. 2018; 17(4): 16–22 (In Russian). https://doi.org/10.20538/1682-0363-2018-4-16-22. EDN: VQWTMH
7. Ruder A.V., Wetzels S.M.W., Temmerman L., et al. Monocyte heterogeneity in cardiovascular disease. Cardiovasc Res. 2023 Sep 5; 119(11): 2033–2045. https://doi.org/10.1093/cvr/cvad069. PMID: 37161473
8. Williams H., Mack C.D., Li S.C.H., et al. Nature versus Number: Monocytes in Cardiovascular Disease. Int J Mol Sci. 2021 Aug 24; 22(17): 9119. https://doi.org/10.3390/ijms22179119. PMID: 34502027
9. Chumakova S.P., Shipulin V.M., Urazova O.I., et al. Ischemic cardiomyopathy: blood monocytes and mediators of their differentiation. Annals of the Russian academy of medical sciences. 2019; 74(6): 396–404 (In Russian). https://doi.org/10.15690/vramn1185. EDN: MOGXIH
10. Felker G.M., Shaw L.K., O’Connor C.M. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002 Jan 16; 39(2): 210–218. https://doi.org/10.1016/s0735-1097(01)01738-7. PMID: 11788209
11. Orozco S.L., Canny S.P., Hamerman J.A. Signals governing monocyte differentiation during inflammation. Curr Opin Immunol. 2021 Dec; 73: 16–24. https://doi.org/10.1016/j.coi.2021.07.007. Epub 2021 Aug 16. PMID: 34411882
12. Pilling D., Fan T., Huang D., et al. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009 Oct 16; 4(10): e7475. https://doi.org/10.1371/journal.pone.0007475. PMID: 19834619
13. Denisenko O.A., Chumakova S.P., Urazova O.I. Endothelial progenitor cells: Origin and role of angiogenesis in cardiovascular diseases. Siberian Journal of Clinical and Experimental Medicine. 2021; 36(2): 23–29 (In Russian). https://doi.org/10.29001/2073-8552-2021-36-2-23-29. EDN: UJDHLQ
14. Chumakova S.P., Urazova O.I., Shipulin V.M., et al. Role of Angiopoietic Coronary Endothelial Dysfunction in the Pathogenesis of Ischemic Cardiomyopathy. Biomedicines. 2023 Jul 10; 11(7): 1950. https://doi.org/10.3390/biomedicines11071950. PMID: 37509589
15. Stelmashenko A.I., Belyaeva S.A., Karpov R.M., et al. Assessment of the extracellular matrix of the myocardium in patients with ischemic cardiomyopathy Morphological almanac named after V.G. Koveshnikov. 2021; 19(4): 65–71 (In Russian). EDN: CPVJHJ
16. Williams H., Mack C., Baraz R., et al. Monocyte differentiation and heterogeneity: inter-subset and interindividual differences. Int J Mol Sci. 2023 May 15; 24(10): 8757. https://doi.org/10.3390/ijms24108757. PMID: 37240103
17. Rogacev K.S., Cremers B., Zawada A.M., et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012 Oct 16; 60(16): 1512–1520. https://doi.org/10.1016/j.jacc.2012.07.019. Epub 2012 Sep 19. PMID: 22999728
18. Peet C., Ivetic A., Bromage D.I., et al. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020 May 1; 116(6): 1101–1112. https://doi.org/10.1093/cvr/cvz336. PMID: 31841135
19. Patel A.A., Zhang Y., Fullerton J.N., et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017 Jul 3; 214(7): 1913–1923. https://doi.org/10.1084/jem.20170355. Epub 2017 Jun 12. PMID: 28606987
20. Shantsila E., Wrigley B., Tapp L., et al. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost. 2011 May; 9(5): 1056–1066. https://doi.org/10.1111/j.1538-7836.2011.04244.x. PMID: 21342432
21. Chen L., Ackerman R., Saleh M., et al. 20-HETE regulates the angiogenic functions of human endothelial progenitor cells and contributes to angiogenesis in vivo. J Pharmacol Exp Ther. 2014 Mar; 348(3): 442–451. https://doi.org/10.1124/jpet.113.210120. Epub 2014 Jan 8. PMID: 24403517
22. Domigan C.K., Warren C.M., Antanesian V., et al. Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J Cell Sci. 2015 Jun 15; 128(12): 2236–2248. https://doi.org/10.1242/jcs.163774. Epub 2015 May 8. PMID: 25956888
About the Authors
M. V. GladkovskayaRussian Federation
Margarita V. Gladkovskaya - Full-Time postgraduate student, Assistant Professor, Pathophysiology Division.
2, Moskovsky Trakt, Tomsk, 634050
S. P. Chumakova
Russian Federation
Svetlana P. Chumakova, Dr. of Sci. (Medicine), Associate Рrofessor, Professor, Pathophysiology Division, Siberian SMU; Professor, Scientific and Educational Center “Dentistry”, Immanuel Kant Baltic Federal University.
2, Moskovsky Trakt, Tomsk, 634050; 14, Alexander Nevsky str., Kaliningrad, 236041
O. I. Urazova
Russian Federation
Olga I. Urazova - Dr. of Sci. (Medicine), Professor, Corresponding Member of RAS, Head of the Pathophysiology Division, Siberian SMU; Leading Researcher, Scientific and Engineering Center «Intelligent Systems of Trusted Interaction», Tomsk SU of Control Systems and Radioelectronics.
2, Moskovsky Trakt, Tomsk, 634050; 40, Lenin Ave., Tomsk, 634050
V. S. Poletika
Russian Federation
Vadim S. Poletika - Cand. of Sci. (Medicine), Associate Professor, Pathophysiology Division.
2, Moskovsky Trakt, Tomsk, 634050
V. M. Shipulin
Russian Federation
Vladimir M. Shipulin - Dr. of Sci. (Medicine), Professor, Honored Scientist of Russia, Chief Researcher, Cardiology Research Institute.
10, Emb. of the river Ushaika, Tomsk, 634050
S. L. Andreev
Russian Federation
Sergey L. Andreev - Cand. of Sci. (Medicine), Cardiovascular Surgeon, Senior Researcher, Cardiovascular Surgery Department, Cardiology Research Institute.
10, Emb. of the river Ushaika, Tomsk, 634050
Supplementary files

|
1. 1137-TREND checklist | |
| Subject | ||
| Type | Исследовательские инструменты | |
Download
(377KB)
|
Indexing metadata ▾ | |
|
|
2. Graphic abstract | |
| Subject | ||
| Type | Research Instrument | |
View
(196KB)
|
Indexing metadata ▾ | |
Review
Журнал "Сеченовский вестник". Лист редактора можно посмотреть здесь /
Sechenov Medical Journal. Editor's checklist you can find here
Название / Title | Фактор роста эндотелия сосудов нивелирует усиление спонтанной трансдифференцировки классических и промежуточных моноцитов при ишемической кардиомиопатии / Vascular endothelial growth factor attenuates enhanced spontaneous transdifferentiation of classical and intermediate monocytes in patients with ischemic cardiomyopathy |
Раздел / Section
| ПАТОЛОГИЧЕСКАЯ ФИЗИОЛОГИЯ / PATHOLOGICAL PHYSIOLOGY |
Тип / Article | Оригинальная статья / Original article |
Номер / Number | 1137 |
Страна/территория / Country/Territory of origin | Россия / Russia |
Язык / Language | Русский / Russian |
Источник / Manuscript source | Инициативная рукопись / Unsolicited manuscript |
Дата поступления / Received | 23.12.2024 |
Тип рецензирования / Type ofpeer-review | Двойное слепое / Double blind |
Язык рецензирования / Peer-review language | Русский / Russian
|
РЕЦЕНЗЕНТ А / REVIEWER A
Инициалы / Initials | 1137_А |
Научная степень / Scientific degree | Доктор медицинских наук / Dr. of Sci. (Medicine) |
Страна/территория / Country/Territory | Россия / Russia |
Дата рецензирования / Date of peer-review | 15.03.2025 |
Число раундов рецензирования / Number of peer-review rounds | 1 |
Финальное решение / Final decision | принять к публикации / accept |
ПЕРВЫЙ РАУНД РЕЦЕНЗИРОВАНИЯ / FIRST ROUND OF PEER-REVIEW
Данная рукопись посвящена актуальной проблеме – социально-значимой патологии – ишемической болезни сердца (ИБС), результаты лечения и профилактики которой до настоящего времени не удовлетворяют клиницистов.
Необратимая форма ИБС имеет высокий риск инвалидизации и неблагоприятных исходов. Выбранные авторами биомаркеры имеют высокую чувствительность к тканевой гипоксии, таким образом даже легкая ишемия может индуцировать их максимальный синтез в раннем периоде. Фенотипическая пластичность моноцитов и макрофагов способствует их привлекательности в качестве терапевтических мишеней.
Представленный материал показывает влияние ключевого фактора неоангиогенеза VEGF-A на изменение субпопуляционного состава моноцитов крови in vitro, что является новым. Результаты исследования in vitro являются первым шагом, позволяющим обозначить возможную новую терапевтическую стратегию. Выводы опираются на полученные результаты и не противоречат данным литературы. Список литературы содержит большинство источников последнего пятилетия.
Статья производит очень приятное впечатление, грамотно разработан дизайн эксперимента, подобраны группы, применяемые методы выделения моноцитов, типирования, культивирования, оценки результатов современны и
адекватны.
На мой взгляд, возможно было бы произвести расчет и обоснование минимально достаточной выборки, что 10–11 человек в каждой из групп достаточно для подтверждения статистической гипотезы.
РЕЦЕНЗЕНТ B / REVIEWER B
Инициалы / Initials | 1137_В |
Научная степень / Scientific degree | Доктор медицинских наук / Dr. of Sci. (Medicine) |
Страна/территория / Country/Territory | Россия / Russia |
Дата рецензирования / Date of peer-review | 28.02.2025 |
Число раундов рецензирования / Number of peer-review rounds | 1 |
Финальное решение / Final decision | принять к публикации / accept |
ПЕРВЫЙ РАУНД РЕЦЕНЗИРОВАНИЯ / FIRST ROUND OF PEER-REVIEW
Так как группа сердечно-сосудистых заболеваний занимает первое место по смертности среди прочих нозологий, тема исследования несомненно очень актуальна. Результаты исследования имеют практическую значимость для повышения эффективности лечения ишемической болезни сердца, в том числе, у пациентов со сниженной фракцией выброса.
Заключение обосновано корректно подобранными современными методами статистической обработки.
Приведенные авторами литературные источники соответствуют цели исследования. В списке из 22 источников приведены 13 источников за последние 5 лет (2020–2023 г. г).
Статья изложена грамотным русским языком, стиль изложения достаточно мягкий, автор не выдвигает безапелляционно свои выводы, а предпочитает рассуждающую манеру изложения, подтверждая сделанное по результатам статьи заключение обоснованными ссылками.