Scroll to:
Bone turnover markers in oral and gingival crevicular fluid in children with end-stage chronic kidney disease
https://doi.org/10.47093/2218-7332.2025.16.1.34-44
Abstract
Objective. To study bone turnover markers in biological fluids (urine, blood serum, oral fluid (OF) and gingival crevicular fluid (GCF)) at the stage of planning an orthodontic strategy in children with end-stage chronic kidney disease (ESKD).
Materials and methods. Pilot, cross-sectional, multicenter study was conducted. A total of 48 children aged 7 to 17 years were examined and divided into three groups: 14 children with ESCKD, 14 children with renal transplant dysfunction (RTD), 20 almost healthy children. Bone turnover markers were assessed by changes in osteocalcin (OC) in the OF, GCF and blood serum, urinary deoxypyridinoline (DPD), levels of total, ionized calcium and phosphorus in blood and pH of OF. Bone tissue mineral density was assessed by cone-beam computerized tomography according to the C. Mish classification.
Results. All groups of children were comparable by gender and age. All patients had no significant mineral and bone disorders. Total and ionized calcium did not demonstrate statistically significant differences between the study groups. Serum phosphorus level was higher in ESCKD children compared to RTD children and control group. Urinary DPD, OC in GCF and OF pH were higher in children with CKD compared to healthy children. However, there were no statistically significant changes between the ESCKD group and the RTD group. In the posterior maxilla, the Hounsfield index was higher in the group with RTD compared to the ESCKD group (p < 0.01), and similar to the control group. In the anterior maxilla, as well as in the anterior and posterior mandibular regions, the Hounsfield index was higher in the control group than in the ESCKD and RTD groups.
Conclusion. The most prominent changes of bone turnover markers were found in children with ESCKD. Urinary DPD and OC in GCF were associated with the decrease in kidney function and jawbone mineral density.
Keywords
Chronic kidney disease (CKD) is a persistent organ damage for three months or more due to various etiologic factors. The pathologic basis of the disease is the process of replacement of normal anatomical structures by fibrosis, which leads to organ dysfunction. CKD is evidenced by a decrease in estimated glomerular filtration rate (eGFR) and/or albuminuria and other markers of kidney damage [1]. The prevalence of CKD in the world population is more than 800 million people [2]. The global mortality from CKD reached 1.2 million in 2017 and is projected to increase [3].
In the world pediatric population, the prevalence of CKD reaches 18.5–58.3 cases per 1 million children [4]. In Russia since 2012, the overall incidence of CKD in children continues to grow [3]. Approaches to CKD diagnosis are unified for both children and adults. However, due to the predominance of non-glomerular etiology of CKD in children, albuminuria is detected less frequently than in adults [5]. According to the European Pediatric Registry, congenital anomalies of the kidney and urinary tract and genetic diseases are the leading etiologic factors of CKD in children, accounting for 40–60% and 20–30% of detected cases, respectively; glomerulonephritis makes an etiologic contribution in less than 10% of cases [5].
End-stage kidney disease (ESCKD) requires renal replacement therapy (hemodialysis, peritoneal dialysis or renal transplant). ESCKD is associated with life quality decline and unfavorable outcomes [6]. Moreover, ESCKD in children is accompanied by significant mineral and bone disorders (CKD-MBD) [7–9], arising as a result of hyperparathyroidism and impaired calcium-phosphorus (Ca-P) metabolism [10][11]. In CKD-MBD children are observed with a decrease in growth [12], a high tendency to fractures [13][14], as well as multiple structural changes in bone tissue, including cortical loss, demineralization, bone trabeculae rarefaction, which are associated with increased osteoclast activity [13].
Bone turnover markers in CKD-MBD are deoxypyridinoline (DPD) and osteocalcin (OC) [15]. DPD is a compound formed during collagen breakdown, it is released into the bloodstream, and then excreted in the urine. DPD reflects osteoclasts activity; DPD level increasing directly correlates to the severity of renal dysfunction in experimental study on rats [16]. OC is a vitamin K-dependent protein synthesized by osteoblasts, reflects impaired bone mineralization in CKD-associated hyperparathyroidism [17][18].
CKD patients are prone to various maxillofacial bone changes such as decreased density of cortical bone and increased jawbone porosity [19], shortened mandible branches, increased gonial angle, decreased posterior facial height [8][20][21], structural and functional temporomandibular joint changes [22][23], along with a significant slowdown in teething [22]. These changes require a personalized approach to orthodontic treatment in CKD children and objective markers for making a medical decision.
Today there are still some open questions about optimal timing for initiating orthodontic treatment and its types in CKD children, and monitoring bone remodeling during this treatment. Thus, searching for biomarkers reflecting specific bone changes including maxillofacial bones in CKD patients remains in demand.
Study objective: to investigate bone turnover markers in different biological fluids (urine, blood serum, oral fluid (OF) and gingival crevicular fluid (GCF) at the stage of planning an orthodontic strategy in children with end-stage chronic kidney disease (ESCKD).
MATERIALS AND METHODS
This pilot cross-sectional multicenter study based on Russian Federal Law No. 323-FZ dated 21.11.2021 “On the Fundamentals of Public Health Protection in the Russian Federation”, (Legislation Bulletin of the Russian Federation, 2011, No. 48, Art. 6724). The required number of patients in groups was determined before the study. The sample size was sufficient given the power of 80%.
Patient enrollment
The study was conducted from March 1 to June 30, 2024, at the following clinical centers: E.V. Borovsky Institute of Dentistry, Sechenov University; Surgical Department No. 1, Academician V.I. Shumakov National Medical Research Center of Transplantology and Artificial Organs, Ministry of Health of the Russian Federation. A continuous recruitment of patients was carried out from those who applied to the above-mentioned medical institutions.
Inclusion criteria:
- age from 7 to 17 years;
- established diagnosis of CKD (ICD-10 codes1: N18 Chronic kidney disease; T86.1 Renal transplant dysfunction);
- dental anomalies, including bite anomalies;
- availability of written informed voluntary consent of parents/legal representatives for the child’s participation in the study.
Non-inclusion criteria:
- active/current orthodontic treatment (n= 5);
- concomitant acute/chronic diseases affecting bone metabolism:
– endocrine and metabolic diseases (n = 10),
– autoimmune diseases (n = 2),
– genetic diseases (n = 4),
– oncological diseases (n = 1),
– diseases of the gastrointestinal tract (n = 3);
– chronic liver diseases (n = 7),
– drug-induced disorders of bone metabolism (n = 5).
A total of 65 children and adolescents were assessed for participation in the study. Exclusion criteria were identified in 37 patients (Fig.). The study included 28 CKD children, who were divided into two groups: Group 1 – 14 ESCKD patients with eGFR according to CKiD U25 with a constant creatinine coefficient ≤ 25 ml/min/1.73m2; Group 2 – 14 patients with kidney graft dysfunction (RTD) with eGFR according to the CKiD U25 with a constant creatinine coefficient > 25 ml/min/1.73 m2.
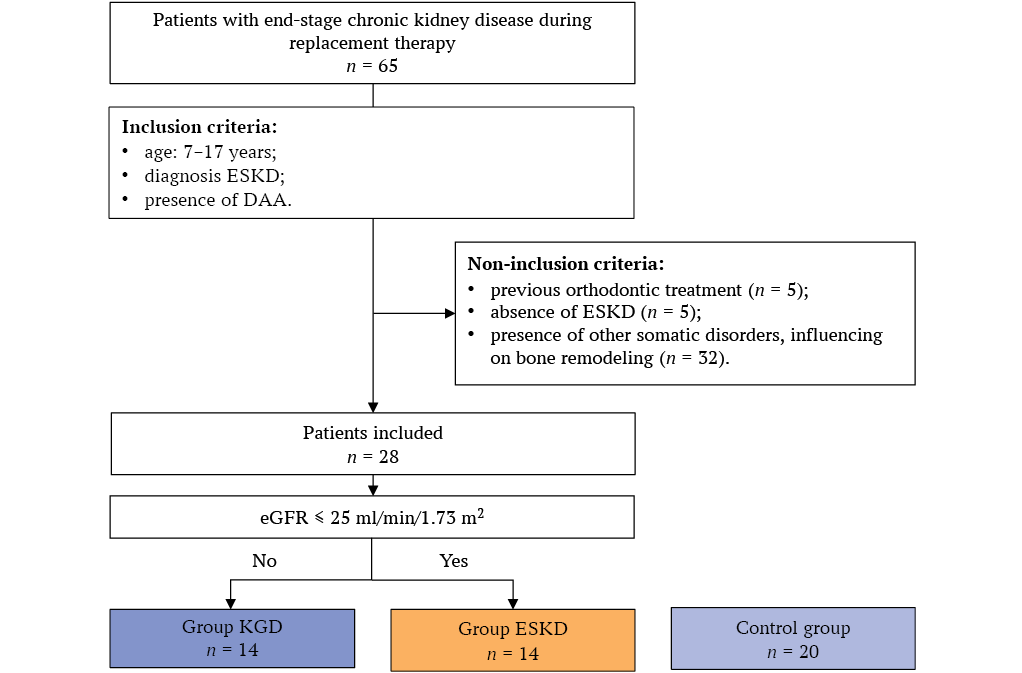
FIG. Study flowchart.
Note: ESCKD – end-stage kidney disease, DAA – dentoalveolar anomalies, RRT – renal replacement therapy, RTD – renal transplant dysfunction, eGFR – estimated glomerular filtration rate (CKiD U25).
The control group consisted of 20 practically healthy children and adolescents with no general medical pathology, matched by sex and age to the group of children with CKD, who underwent dental examination at the Department of Pediatric, Preventive Dentistry and Orthodontics in E.V. Borovsky Institute of Dentistry during the study period.
Determination of bone metabolism biomarkers
Biological fluids from patients were taken once in the morning before the breakfast and diagnostic and therapeutic procedures. Blood samples of 5 mL each were collected from cubital vein or from the hand back veins, stabilized with heparin (25 IU/ml), urine sample of 50 mL each, oral fluid sample at least 5 mL.
Biochemical blood analysis was performed by photocolorimetric methods to determine the level of total (Ca) and ionized (Ca2+) calcium, phosphorus (P). Calculation of total blood plasma calcium with correction for albumin was performed according to the formula: measured plasma calcium level (mmol/L) + 0.02*(40 – measured plasma albumin level (g/L).
Urinary DPD was measured by solid-phase chemiluminescent immunoassay.
OC in serum, GCF and OF was measured by using commercial Osteocalcin ELISA kits for solid-phase enzyme-linked immunosorbent assay (BioVendor, USA).
The Milwaukee PH56 (Milwaukee Instruments, USA) device was used to determine oral pH.
Bone density was assessed using cone-beam computed tomography (CBCT), expressed in Hounsfield units (HU) according to C. Mish classification [24], based on the mathematical reconstruction of X-ray attenuation coefficients assigned to each pixel. The X-ray assessment was performed in four sections: the anterior and posterior sections of the upper jaw; the anterior and posterior sections of the lower jaw.
Statistical analysis
Quantitative features are presented as median and interquartile range, qualitative ones – as proportion. The studied features of patient groups were tested for normal distribution using Shapiro-Wilk test and for homogeneity of variances using Levene test. Variables corresponding to normal distribution and having homogeneous variances are presented as mean values and standard deviation, mean values were compared using one-way analysis of variance (ANOVA). Other variables are presented as median and interquartile range (25th; 75th percentiles), for their comparison the Kruskal–Wallis method was used. For post-hoc analysis the Tukey test was used. The results of statistical analysis were considered significant at p < 0.05. The experimental results were processed using Prism 8.0.1 (GraphPad Software, USA) and R language. 4.4.2 in the R-Studio software environment.
RESULTS
The study included patients without pronounced clinical manifestations of CKD-MBD and osteoporosis. The main characteristics of the study groups are presented in Table 1.
Mean age in study groups were 12.7 ± 2.9 years and girls’ number in RTD group was lower than in ESCKD group and control, but the differences were not statistically significant (Table 1).
Serum creatinine in ESCKD group was significantly higher and eGFR was lower compared to RTD group and control. ESCKD patients received hemodialysis or peritoneal dialysis treatment for an average from 6 months to 3 years.
Total and ionized serum calcium did not differ between the study groups (Table 1). Serum P was significantly higher in ESCKD group compared to RTD group and control. While there were not found any statistically significant differences in phosphorus levels in RTD group and control (Table 1).
Summarized markers results in studied groups are presented in Table 2.
Urinary DPD concentration was higher in CKD groups compared to control (Table 2). However, no statistically significant differences were found between urinary DPD concentration in ESCKD and RTD patients.
Serum OC concentration was increased in ESCKD patients compared to control (p < 0.05) and did not differ from RTD group. OC in GCF was higher in control compared to ESCKD (p < 0.001) and RTD (p < 0.001) groups. Meanwhile salivary OC was comparable in all groups (Table 2).
OF pH was statistically significantly higher in both ESCKD and RTD children compared to control (Table 2). Moreover, there were no differences between oral pH in RTD and ESCKD groups, thus oral acidity was similar in these two groups.
Bone density radiographical assessment shows that Hounsfield Index of posterior maxilla was higher in RTD group compared to ESCKD group (p < 0.01), and there was no difference with control. Hounsfield Index of anterior maxilla in control was higher than in ESCKD and in RTD groups. A similar pattern was found in Hounsfield index of both anterior and posterior mandible where the control group levels were statistically significantly higher than in ESCKD and RTD patients (Table 2).
Table 1. Characteristics of study patient groups
|
Feature |
Chronic kidney disease |
Control group (n = 20) |
p value (ANOVA) |
|
|
ESCKD (n = 14) |
RTD (n = 14) |
|||
|
Age, years |
12.1 ± 2.4 |
13.4 ± 3.0 |
12.6 ± 3.4 |
n.s. |
|
Girls, n (%) |
11 (79) |
6 (43) |
13 (65) |
n.s. |
|
eGFR ml/min/1.73 m² |
10.51 ± 3.25 a,c |
56.73 ± 15.31 b,c |
90.01 ± 10.26 a,b |
<0.0001 |
|
Creatinine in serum, µmol/L |
477.8 (403.1; 571.6) a,c |
85.7 (73.2; 131.9) b,c |
63.0 (50.35; 71.68) a,b |
<0.0001 |
|
Calcium total in serum, mmol/L |
2.40 (2.14; 2.62) |
2.42 (2.34; 2.46) |
2.40 (2.27; 2.49) |
<0.01 |
|
Total serum calcium adjusted for albumin, mmol/L |
2.34 ± 0.28 |
2.39 ± 0.13 |
2.37 ± 0.14 |
n.s. |
|
Calcium ionized in serum, mmol/L |
1.16 (0.96; 1.21) |
1.18 (1.10; 1.23) |
1.21 (1.17; 1.24) |
<0.01 |
|
Phosphorus in serum, mmol/L |
1.751 ± 0.490 a,c |
1.342 ± 0.266 s |
1.436 ± 0.195 a |
<0.005 |
Note: RTD – renal transplant dysfunction; eGFR – estimated glomerular filtration rate; ESCKD – end-stage chronic kidney disease; a – p < 0.05 when comparing ESCKD and control groups; b – p < 0.05 when comparing RTD and control groups; c – p < 0.05 when comparing RTD and ESCKD.
Table 2. Bone turnover markers
|
Feature |
Chronic kidney disease |
Control group (n = 20) |
p value (ANOVA) |
|
|
ESCKD (n = 14) |
RTD (n = 14) |
|||
|
Urinary DPD, nmol/mmolCreat |
15.80 (12.68; 27.90) a |
15.08 (10.27; 24.61) b |
4.90 (2.95; 11.98) a,b |
<0.001 |
|
Serum OC, ng/mL |
213.1 ± 55.01 a |
173.7 ± 86.78 |
153.9 ± 56.15 a |
<0.05 |
|
Salivary OC, ng/mL |
11.78 ± 1.93 |
12.94 ± 1.76 |
13.46 ± 3.73 |
n.s. |
|
ОС in gingival crevicular fluid, ng/mL |
13.11 ± 3.98 a |
11.92 ± 3.10 b |
20.08 ± 4.69 a,b |
<0.0001 |
|
Oral fluid рН |
7.080 (6.375; 8.153) a |
7.240 (6.875; 7.593) b |
6.250 (5.575; 6.800) a.b |
<0.001 |
|
Hounsfield Index of anterior maxilla |
482.5 (394.5; 554.3) a |
439.0 (396.3; 503.0) b |
681.5 (449.0; 766.8) a.b |
<0.0001 |
|
Hounsfield Index of posterior maxilla |
203.0 (194.8; 238.8) a.c |
363.0 (248.3; 485.0) c |
420.0 (329.0; 539.0) a |
<0.01 |
|
Hounsfield Index of anterior mandible |
1059 (951; 1451) a |
1670 (1083; 1985) b |
3098 (1985; 3538) a.b |
<0.0001 |
|
Hounsfield Index of posterior mandible |
824.4 ± 111.0 a |
826.5 ± 89.5 b |
1735 ± 377.2 a.b |
<0.0001 |
Note: DPD – deoxypyridinoline; RTD – renal transplant dysfunction; ESCKD – end-stage chronic kidney disease; OC – osteocalcin; max – maxilla; man – mandible; a – p < 0.05 when comparing ESCKD and control groups; b – p < 0.05 when comparing RTD and control groups; c – p < 0.05 when comparing RTD and ESCKD.
DISCUSSION
Study results demonstrated CBCT changes in bone turnover markers and bone density were the most pronounced in ESCKD children. Urinary DPD increase, a decrease in serum and GCF OC and a decrease in the Hounsfield index in both anterior and posterior regions of the maxilla and mandible were revealed. Similar changes were noted in RTD patients, but OC level was reduced only in GCF. Bone density assessment had no significant differences between ESCKD and RTD groups, except Hounsfield index in posterior maxilla.
Kidneys play a crucial role in Ca-P metabolism by almost complete tubular reabsorption of these ions. Ca-P homeostasis in CKD patients is disrupted, so serum P in ESCKD patients had been increasing with eGFR decreasing in our study. It’s important to check serum P in CKD-MBD patients for maintaining bone homeostasis, and our results are consistent with Rastogi A. et al. [25]. Serum P in KDG group was close to control group, and inversely correlates with a higher eGFR level, because of kidney filtration improvement after transplantation, but due graft dysfunction P level remained high. These trends are consistent with other studies data on mineral metabolism effect on bone remodeling in patients after kidney transplantation [26][27].
Our study results showed that serum Ca does not statistically differ between the groups, which indicates the stability of this marker regardless of kidney and graft function. However, ESCKD markers are less homogeneous and it still has a greater spread. No expressed calcium metabolism disorders were detected as well as in Liu J. et al. and Hasanzamani B. et al. studies [28][29]. It should be noted that the levels of parathyroid hormone and bone fraction of alkaline phosphatase were not taken into account.
Urinary DPD was found as highly sensitive marker of bone metabolism disorders in ESCKD and RTD patients. Thus, we found clear and significant differences in this marker between the study groups. DPD increase in CKD children compared to control may indicate high osteoclast activity and bone resorption activation. DPD changes were recorded simultaneously with a Hounsfield index decrease in jawbones. It indicates bone collagen breakdown, type I mainly, the end products of bone metabolism excretion with urine and it confirms persistent bone metabolism changes in CKD children. However, literary data did not confirm that urinary DPD can reflect bone metabolism in CKD-MBD patients [30]. On the other hand, DPD level is known as one of the leading biochemical markers of bone remodeling and is used in osteoporosis early diagnosis [31]. Thus, urinary DPD determination in CKD patients may become promising for assessing osteoclasts activity and bone resorption and requires further study on a larger CKD patient sample.
Presented study results convincingly demonstrate that OC is an informative marker of osteoblast activity in ESCKD and RTD children, and GCF is the best biological fluid for its determination, since the most significant OC changes are registered in GCF, despite the limited sample. OC decrease in GCF was found in ESCKD and RTD children compared to control, which indicates a violation of bone metabolism. Serum OC increase was detected only in ESCKD group probably due to the limited sample. OC in OF did not statistically significantly differ between three groups possibly due to high protease activity in OF [32]. We didn’t find any information about OC measurement in GCF in CKD patients in the reviewed literature. However, Fadli N. et al. used GCF for the assessment of OC and proinflammatory markers [33]. Interest in GCF exertion as a fluid for various markers detection in patients with systemic diseases, including CKD, is growing due to its sufficient informativeness and minimally invasive nature.
OF pH increase with its alkaline tendency may be associated with a disturbance in general metabolism, including a change in the acid-base balance in ESCKD patients [34].
In addition, the obtained data indicate significant disturbances in bone structure in ESCKD and RTD children, which is manifested in a significant decrease in Hounsfield index compared to control, especially in the anterior and posterior mandible. These changes are consistent with previous studies [23], confirming disturbances in bone metabolism and decreased bone mineralization in patients with renal dysfunction.
Limitations and directions for future research
Result interpretations have several limitations due to pilot study design: small sample size, one observation point. The levels of parathyroid hormone and bone fraction of alkaline phosphatase were not taken into account when assessing CKD-MBD. Perhaps due to insufficient study power, there were no statistically significant differences in total serum calcium adjusted for albumin, as well as OC in oral fluid. To draw conclusions on these markers, it is necessary to conduct longitudinal studies on large samples using probability selection of observation units.
Ethics statements. This study using biological material was conducted in accordance with the World Medical Association's Declaration of Helsinki on Ethical Principles of Biomedical Research. The study was conducted in accordance with the permission of the Local Ethics Committee of I.M. Sechenov First Moscow State Medical University of the Russian Ministry of Health (Sechenov University), No. 01-22 dated January 20, 2022. Informed voluntary consent for inclusion in the study was obtained from one of the patient's parents or other legal representative.
Data access. The data that support the findings of this study are available from the corresponding authors upon reasonable request. The data and statistical methods presented in the article have been statistically reviewed by the journal editor, a certified biostatistician.
Conflict of interest. The authors declare that there is no conflict of interest.
Financing. The study had no sponsorship (own resources).
Соответствие принципам этики. Данное исследование с использованием биологического материала проводилось в соответствии с Хельсинкской декларацией Всемирной медицинской ассоциации об этических принципах проведения биомедицинских исследований. Исследование проведено в соответствии с разрешением Локального этического комитета ФГАОУ ВО «Первый МГМУ им. И.М. Сеченова» Минздрава России (Сеченовский Университет) (№ 01-22 от 20.01.2022). Информированное добровольное согласие на включение в исследование было получено у одного из родителей или иного законного представителя пациента.
Доступ к данным исследования. Данные, подтверждающие выводы этого исследования, можно получить у авторов по обоснованному запросу. Данные и статистические методы, представленные в статье, прошли статистическое рецензирование редактором журнала – сертифицированным специалистом по биостатистике.
Конфликт интересов. Авторы заявляют об отсутствии конфликта интересов.
Финансирование. Исследование не имело спонсорской поддержки (собственные ресурсы).
1 International Classification of Diseases, 10th revision (ICD-10). Access date: 10.01.2025 . https://mkb-10.com
References
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024 Apr; 105(4S): S117–S314. https://doi.org/10.1016/j.kint.2023.10.018. PMID: 38490803
2. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022 Apr; 12(1): 7–11. https://doi.org/10.1016/j.kisu.2021.11.003. Epub 2022 Mar 18. PMID: 35529086
3. Rumyantseva E.I. Сhronic kidney disease as a global public health problem: trends in morbidity and mortality. 2021; 1–2: 41–49 (In Russian). https://doi.org/10.26347/1607-2502202101-02041-049. EDN: TFHKJB
4. Аxmedova E.A. Сhronic kidney disease in children (literature review). Journal of clinical and preventive medicine. 2024;1(1):94– 98 (In Russian). ISSN 2181-3531
5. Harambat J., van Stralen K.J., Kim J.J., Tizard E.J. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012 Mar; 27(3): 363–373. https://doi.org/10.1007/s00467-011-1939-1. Epub 2011 Jun 29. Erratum in: Pediatr Nephrol. 2012 Mar; 27(3): 507. PMID: 21713524
6. Borzych D., Rees L., Ha I.S., et al. The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney Int. 2010 Dec; 78(12): 1295–1304. https://doi.org/10.1038/ki.2010.316. Epub 2010 Sep 1. PMID: 20811335
7. Melo V.B., Silva D.B.D., Soeiro M.D., et al. Growth in children with chronic kidney disease and associated risk factors for short stature. J Bras Nefrol. 2024 Oct-Dec; 46(4): e20230203. https://doi.org/10.1590/2175-8239-JBN-2023-0203en. PMID: 39094068
8. Simic P. Bone and bone derived factors in kidney disease. Front Physiol. 2024 Mar 1;15:1356069. https://doi.org/10.3389/fphys.2024.1356069. PMID: 38496297
9. Karlovich N.V., Mokhort T.V., Sazonava A.G. Bone pathology in chronic kidney disease. Osteoporosis and Bone Diseases. 2022; 25(1): 29–38 (In Russian). https://doi.org/10.14341/osteo12943. EDN: EOULOC
10. Elhusseiny G.A., Saleh W. Oral health in children with chronic kidney disease, hemodialysis, and renal transplantation: a comprehensive narrative review of the oral manifestations and dental implications. Clin Med Insights Pediatr. 2024 Aug 27; 18: 11795565241271689. https://doi.org/10.1177/11795565241271689. PMID: 39206206
11. Denburg M.R., Kumar J., Jemielita T., et al. Fracture burden and risk factors in childhood CKD: results from the CKiD cohort study. J Am Soc Nephrol. 2016 Feb; 27(2): 543–550. https://doi.org/10.1681/ASN.2015020152. Epub 2015 Jul 2. PMID: 26139439
12. Printza N., Dotis J., Sinha M.D., Leifheit-Nestler M. Editorial: Mineral and bone disorder in CKD. Front Pediatr. 2022 Feb 18; 10: 856656. https://doi.org/10.3389/fped.2022.856656. PMID: 35252071
13. Todisco T., Ubertini G.M., Bizzarri C., et al. Chronic kidney disease and growth failure in children. Children (Basel). 2024 Jul 1; 11(7): 808. https://doi.org/10.3390/children11070808. PMID: 39062256
14. Chi P.J., Hung S.Y., Hsiao F.T., et al. Serum osteocalcin concentration as an independent biomarker of osteoporosis in patients with chronic kidney disease. Clin Nephrol. 2022 Jul; 98(1): 1–9. https://doi.org/10.5414/CN110705. PMID: 35445659
15. Heimgartner N., Graf N., Frey D., et al. Predictive power of bone turnover biomarkers to estimate bone mineral density after kidney transplantation with or without denosumab: a post hoc analysis of the POSTOP study. Kidney Blood Press Res. 2020; 45(5): 758–767. https://doi.org/10.1159/000510565. Epub 2020 Sep 30. PMID: 32998144
16. Ziemińska M., Pawlak D., Sieklucka B., et al. Vitamin K-dependent carboxylation of osteocalcin in bone-ally or adversary of bone mineral status in rats with experimental chronic kidney disease? Nutrients. 2022 Oct 1; 14(19): 4082. https://doi.org/10.3390/nu14194082. PMID: 36235734
17. Tsugawa N., Shiraki M. Vitamin K Nutrition and Bone Health. Nutrients. 2020 Jun 27; 12(7): 1909. https://doi.org/10.3390/nu12071909. PMID: 32605143
18. Mohamed FF., Amadeu de Oliveira F., Kinoshita Y., et al. Dentoalveolar alterations in an adenine-induced chronic kidney disease mouse model. J Bone Miner Res. 2023 Aug; 38(8): 1192–1207. https://doi.org/10.1002/jbmr.4829. Epub 2023 May 27. PMID: 37191192
19. Lalayiannis A.D., Soeiro E.M.D., Moysés R.M.A., Shroff R. Chronic kidney disease mineral bone disorder in childhood and young adulthood: a ‘growing’ understanding. Pediatr Nephrol. 2024 Mar; 39(3): 723–739. https://doi.org/10.1007/s00467-023-06109-3. Epub 2023 Aug 25. PMID: 37624528
20. Munagala K.K., Nanda S., Chowdhary Z., et al. Severity of periodontal disease in chronic kidney disease patients: a hospitalbased study. Cureus. 2022; 14(6): e25646. Published 2022 Jun 3. https://doi.org/10.7759/cureus.25646
21. Morozova N.S., Mamedov A.A., Lakomova D.Y., et al. Long-term changes in the dentoalveolar system of rats after experimental intra-abdominal hypertension. Sechenov Medical Journal. 2021; 12(3): 38–46 (In Russian). https://doi.org/10.47093/2218-7332.2021.12.3.38-46. EDN: VDAIPF
22. Morozova O.L., Morozova N.S., Mamedov А.А., et al. Changes in the dentoalveolar system in children with chronic kidney disease. Pediatria n.a. G.N. Speransky. 2018; 97(5): 104–112 (In Russian). https://doi.org/10.24110/0031403X-2018-97-5-104-112. EDN: XZIRU
23. Morozova N.S., Elovskaya A.A., Timoshchenko T.V. et al. Orthodontic rehabilitation of a patient with chronic kidney disease after transplantation. Vrach (The Doctor) 2021; 32(10): 50–53 (In Russian). https://doi.org/10.29296/25877305-2021-10-09. EDN: YPDDTM
24. Misch C.E., Judy K.W. Classification of partially edentulous arches for implant dentistry. Int J Oral Implantol. 1987; 4(2): 7–13. PMID: 3269839
25. Rastogi A, Bhatt N, Rossetti S, Beto J. Management of hyperphosphatemia in end-stage renal disease: a new paradigm. J Ren Nutr. 2021 Jan; 31(1): 21–34. https://doi.org/10.1053/j.jrn.2020.02.003. Epub 2020 May 5. PMID: 32386937
26. Cseprekál O., Kis E., Dégi A.A., et al. Bone metabolism and arterial stiffness after renal transplantation. Kidney Blood Press Res. 2014; 39(6): 507–515. https://doi.org/10.1159/000368461. Epub 2014 Nov 28. PMID: 25531154
27. Bellorin-Font E., Rojas E., Martin K.J. Bone disease in chronic kidney disease and kidney transplant. Nutrients. 2022 Dec 29; 15(1): 167. https://doi.org/10.3390/nu15010167. PMID: 36615824
28. Liu J., Tio M.C., Verma A., et al. Determinants and outcomes associated with urinary calcium excretion in chronic kidney disease. J Clin Endocrinol Metab. 2022 Jan 1; 107(1): e281–e292. https://doi.org/.1210/clinem/dgab574. PMID: 34390334
29. Hasanzamani B., Karimi N., Sabbagh M.G., Majd H.M. The relationship between pre-transplant serum phosphorus before kidney transplantation with early graft dysfunction. Iran J Kidney Dis. 2021 Mar; 1(2): 148–154. PMID: 33764326
30. Coen G., Mantella D., Calabria S., et al. Urinary deoxypyridinoline excretion for the evaluation of bone turnover in chronic renal failure. Am J Nephrol. 2000 Jul-Aug; 20(4): 283–290. https://doi.org/10.1159/000013602. PMID: 10970981
31. Abdelfattah Abulfadle K., Refaat Abdelkader Atia R., Osama Mohammed H., et al. The potential anti-osteoporotic effect of exercise-induced increased preptin level in ovariectomized rats. Anat Sci Int. 2023 Jan; 98(1): 22–35. https://doi.org/10.1007/s12565-022-00666-7. Epub 2022 May 4. PMID: 35507276
32. Tavares L.T.R., Saavedra-Silva M., López-Marcos J.F., et al. Blood and salivary inflammatory biomarkers profile in patients with chronic kidney disease and periodontal disease: a systematic review. Diseases. 2022 Feb 17; 10(1): 12. https://doi.org/10.3390/diseases10010012. PMID: 35225864
33. Fadli N.A., Abdul Rahman M., Karsani S.A., Ramli R. Oral and gingival crevicular fluid biomarkers for jawbone turnover diseases: a scoping review. Diagnostics (Basel). 2024 Sep 30; 14(19): 2184. https://doi.org/10.3390/diagnostics14192184. PMID: 39410587
34. Rodrigues R.P.C.B., Vidigal M.T.C., Vieira W.A., et al. Salivary changes in chronic kidney disease and in patients undergoing hemodialysis: a systematic review and meta-analysis. J Nephrol. 2022 Jun; 35(5): 1339–1367. https://doi.org/10.1007/s40620-022-01274-4. Epub 2022 Mar 2. PMID: 35235185
About the Authors
A. A. ElovskayaRussian Federation
Alina A. Elovskaya - Assistant Professor, Pediatric, Preventive dentistry and Orthodontics Department in E.V. Borovsky Institute of Dentistry.
8/2, Trubetskaya str., Moscow, 119048
E. A. Maslikova
Russian Federation
Ekaterina A. Maslikova - Assistant Professor, Pediatric, Preventive dentistry and Orthodontics Department in E.V. Borovsky Institute of Dentistry.
8/2, Trubetskaya str., Moscow, 119048
N. S. Morozova
Russian Federation
Natalia S. Morozova - Dr. of Sci. (Medicine), Professor of the Department of Dental Diseases Propaedeutics in E.V. Borovsky Institute of Dentistry.
8/2, Trubetskaya str., Moscow, 119048
N. B. Zakharova
Russian Federation
Natalia B. Zakharova - Dr. of Sci. (Medicine), Professor, Department of Clinical Laboratory Diagnostics.
112, Bolshaya Kazachia str., Saratov, 410012
L. D. Maltseva
Russian Federation
Larisa D. Maltseva - Cand. of Sci. (Medicine), Associate Professor, Pathophysiology Department, Sechenov First.
8/2, Trubetskaya str., Moscow, 119048
E. Yu. Danilova
Russian Federation
Elena Y. Danilova - Junior research assistant of the Laboratory of Molecular Modeling and Chemistry of Natural Compounds of the Institute of Molecular Theranostics of the Science and Technology Park of Medicine.
8/2, Trubetskaya str., Moscow, 119048
I. I. Shaikhattarova
Russian Federation
Ilsiiar I. Shaikhattarova - student, E.V. Borovsky Institute of Dentistry.
8/2, Trubetskaya str., Moscow, 119048
A. A. Shirina
Russian Federation
Angelina А. Shirina - student, E.V. Borovsky Institute of Dentistry.
8/2, Trubetskaya str., Moscow, 119048
V. A. Shustova
Russian Federation
Violetta A. Shustova - student, E.V. Borovsky Institute of Dentistry.
8/2, Trubetskaya str., Moscow, 119048
O. L. Morozova
Russian Federation
Olga L. Morozova - Dr. of Sci. (Medicine), Professor, Pathophysiology Department.
8/2, Trubetskaya str., Moscow, 119048
Supplementary files

|
1. STROBE Statement—Checklist | |
| Subject | ||
| Type | Исследовательские инструменты | |
Download
(199KB)
|
Indexing metadata ▾ | |
|
|
2. Graphic abstract | |
| Subject | ||
| Type | Research Instrument | |
View
(145KB)
|
Indexing metadata ▾ | |
Review
Журнал "Сеченовский вестник". Лист редактора можно посмотреть здесь /
Sechenov Medical Journal. Editor's checklist you can find here
Название / Title | Маркеры ремоделирования костной ткани в ротовой и зубодесневой жидкостях у детей с терминальной стадией хронической болезни почек / Bone turnover markers in oral and gingival crevicular fluid in children with end-stage chronic kidney disease |
Раздел / Section
| ПАТОЛОГИЧЕСКАЯ ФИЗИОЛОГИЯ / PATHOLOGICAL PHYSIOLOGY |
Тип / Article | Оригинальная статья / Original article |
Номер / Number | 1170 |
Страна/территория / Country/Territory of origin | Россия / Russia |
Язык / Language | Русский / Russian |
Источник / Manuscript source | Инициативная рукопись / Unsolicited manuscript |
Дата поступления / Received | 17.01.2025 |
Тип рецензирования / Type ofpeer-review | Двойное слепое / Double blind |
Язык рецензирования / Peer-review language | Русский / Russian
|
РЕЦЕНЗЕНТ А / REVIEWER A
Инициалы / Initials | 1170_А |
Научная степень / Scientific degree | Доктор медицинских наук / Dr. of Sci. (Medicine) |
Страна/территория / Country/Territory | Россия / Russia |
Дата рецензирования / Date of peer-review | 17.02.2025 |
Число раундов рецензирования / Number of peer-review rounds | 1 |
Финальное решение / Final decision | принять к публикации / accept |
ПЕРВЫЙ РАУНД РЕЦЕНЗИРОВАНИЯ / FIRST ROUND OF PEER-REVIEW
Актуальность исследования не вызывает сомнений. Заболеваемость хронической болезнью почек (ХБП) у детей растет как в мире, так и в России. Известно, что ХБП сопровождается минерально-костными нарушениями вследствие нарушения фосфорно-кальциевого обмена. По данным литературы у таких больных наблюдаются проблемы с развитием скелета, склонность к переломам, нарушения структуры костной ткани. У пациентов с ХБП отмечаются, в том числе, и изменения костной ткани челюстно-лицевой области, замедляется процесс прорезывания зубов. При этом необходимо учитывать, что на терминальной стадии ХБП детям требуется проведение заместительной почечной терапии, что сопровождается дальнейшим ухудшением качества жизни и затрудняет лечение сопутствующей патологии.
Авторы поставили своей целью решить непростые вопросы об оптимизации и выборе оптимальных сроков начала ортодонтического лечения у таких детей, а также мониторирования процесса костного ремоделирования на фоне лечения ХБП. Для этого необходимы актуальные биомаркеры, четко и быстро показывающие специфические изменения в костной ткани. Поиск таких маркеров и разработка персонализированного подхода определяет научную новизну работы и несомненную практическую значимость.
Материалы и методы полностью адекватны поставленной цели. Авторами проведено пилотное проспективное одномоментное многоцентровое диагностическое исследование, направленное на анализ изменений маркеров костного метаболизма в различных биологических жидкостях до начала ортодонтического лечения, у детей с терминальной стадией ХБП и детей с дисфункцией трансплантата почки. Изучены показатели и у группы сравнения - практически здоровых детей и подростков без патологии мочевыделительной системы. Возраст и пол исследуемых пациентов сопоставим. Использованы адекватные методы исследования, проведена корректная статистическая обработка. Понятно представлен дизайн исследования, описаны критерии невключения в работу.
Полученные данные корректно описаны. Заключение соответствует поставленной цели. Наиболее значимые изменения маркеров ремоделирования кости выявлены у детей с терминальной ХБП. Определены перспективным для оценки ремоделирования кости маркеры.
Установленные сдвиги маркеров костного метаболизма подтверждают необходимость в разработке стратегии персонифицированного и мультидисциплинарного подхода к ортодонтическом лечению данной когорты пациентов.
Авторы в своей работе пользовались научной терминологией, полностью принятой в таких областях науки как патологическая физиология, стоматология, нефрология, клиническая лабораторная диагностика. Список использованной литературы валиден. Из представленных 37 литературных источников 32 - глубиной до пяти лет.
Работа представляет собой целостный законченный текст, разделы логичны, не противоречат друг другу. Стиль изложения понятный, применяемый английский язык представлен на достойном уровне.
РЕЦЕНЗЕНТ B / REVIEWER B
Инициалы / Initials | 1170_В |
Научная степень / Scientific degree | Доктор медицинских наук / Dr. of Sci. (Medicine) |
Страна/территория / Country/Territory | Россия / Russia |
Дата рецензирования / Date of peer-review | 21.02.2025 |
Число раундов рецензирования / Number of peer-review rounds | 1 |
Финальное решение / Final decision | принять к публикации / accept |
ПЕРВЫЙ РАУНД РЕЦЕНЗИРОВАНИЯ / FIRST ROUND OF PEER-REVIEW
Актуальность цели выполненного исследования достаточно высокая. Научная новизна и практическая значимость представленных результатов не вызывает сомнений.
Использованные в работе материалы и методы, вполне адекватны поставленной цели, статистическая обработка полученных данных соответствует их характеру.
Заключение в статье обосновано, сделано на основе глубокого и всестороннего анализа полученных результатов с использованием информативных и актуальных литературных источников.
Вся научная терминология в статье использована правильно и полностью соответствует принятой в рассматриваемой области знаний.
Использованные источники литературы валидные, современные, их цитирование является обоснованным и не вызывает сомнений.
Текст статьи является целостным и не содержит противоречий. Статья написана профессионально, академично, хорошим литературным языком, что существенно облегчает его восприятие и понимание сути работы.
В целом, представленная статья выполнена на высоком методическом уровне на актуальную тему, обладает научной новизной и высокой практической значимостью, ее результаты достаточно четко обоснованы и конкретны.







































