Scroll to:
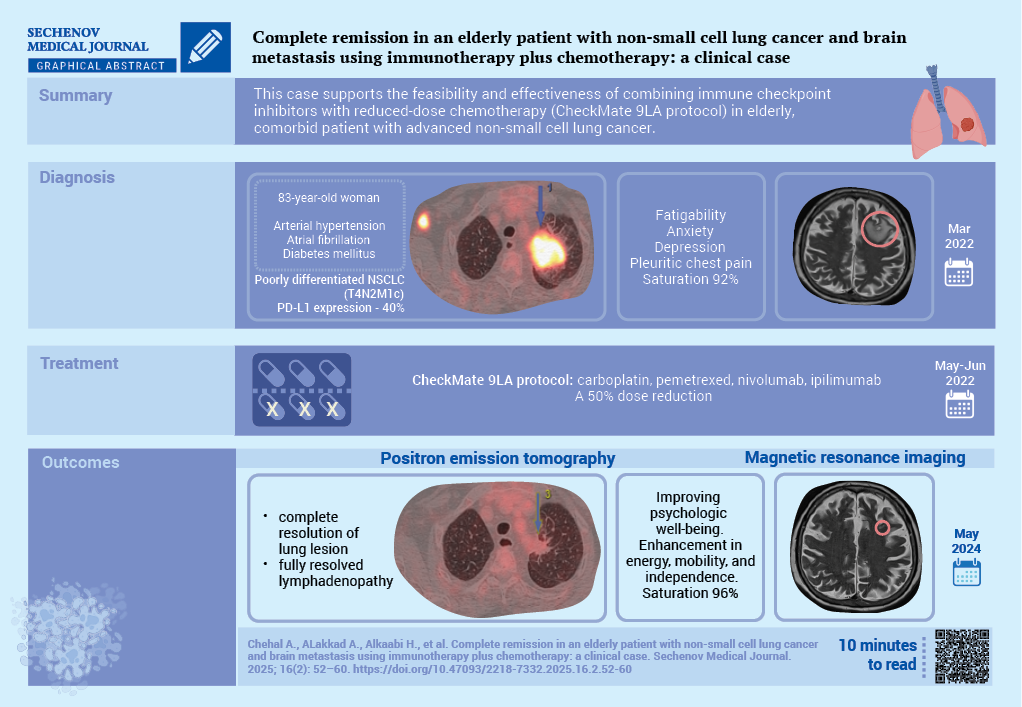
Complete remission in an elderly patient with non-small cell lung cancer and brain metastasis using immunotherapy plus chemotherapy: a clinical case
https://doi.org/10.47093/2218-7332.2025.16.2.52-60
Abstract
Lung cancer remains a leading cause of cancer-related mortality, with non-small cell lung cancer (NSCLC) accounting for the majority of cases. Among its subtypes, adenocarcinoma is most prevalent. Stage IV NSCLC comes with a poor prognosis, particularly in elderly patients with comorbidities. Programmed death-ligand 1 (PD-L1) checkpoint inhibitors have demonstrated promising efficacy, including in cases with brain metastases.
Case report. The case concerns an 83-year-old woman with diabetes mellitus, arterial hypertension, and atrial fibrillation, diagnosed with stage IVB poorly differentiated lung adenocarcinoma which was confirmed by a percutaneous lung biopsy. PD-L1 expression was 40%. Magnetic resonance imaging identified a solitary brain metastasis. The patient was treated with dexamethasone and a CheckMate 9LA protocol was initiated with reduced-dose carboplatin, pemetrexed, nivolumab, and ipilimumab. A two years follow-up positron emission tomography showed a significant reduction in lung cancer. The brain lesions had almost disappeared, and in addition a clinical improvement could be observed.
Discussion. This case underscores the potential for durable remission and improved quality of life through individualized treatment strategies in older patients with advanced NSCLC and brain involvement
Keywords
Abbreviations:
- ICIs – immune checkpoint inhibitors
- NSCLC – non-small cell lung cancer
- PD-L1 – programmed death-ligand 1
Lung cancer accounts for approximately 12% of all malignancies worldwide, with non-small cell lung cancer (NSCLC) representing nearly 80% of all cases [1][2]. Histopathologically, NSCLC encompasses several subtypes, including adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and large cell carcinoma [3]. The 2015 World Health Organization classification introduced significant refinements, such as revised criteria for adenocarcinoma, subdivision of squamous cell carcinoma into keratinizing and non-keratinizing (basaloid) types, and a narrowed definition for large cell carcinoma. Neuroendocrine tumors were grouped under a unified framework, and a more nuanced grading approach was adopted [4].
The 2021 World Health Organization update further expanded molecular testing recommendations, reflecting the growing importance of precision oncology1. While the 2015 guidelines emphasized testing for epidermal growth factor receptor mutations and anaplastic lymphoma kinase rearrangements, the 2021 edition included additional targets such as RET, ROS1, KRAS, MET, NTRK1–3, ERBB2, and BRAF, alongside programmed death-ligand 1 (PD-L1) expression assessment by tumor proportion score or combined positive score [4][5].
Brain metastasis remains a major complication in NSCLC, contributing to morbidity and reduced overall survival [6]. Approximately 25% of patients with epidermal growth factor receptor – mutant NSCLC present with central nervous system involvement at diagnosis, and this rate exceeds 45% within three years despite treatment with epidermal growth factor receptor tyrosine kinase inhibitors [7]. Traditional management options for limited brain metastases have included surgical resection, whole-brain radiotherapy, and stereotactic radiosurgery [8]. However, the poor prognosis associated with central nervous system involvement has led to increased interest in systemic immunotherapy, particularly immune checkpoint inhibitors (ICIs) [8].
ICIs, alone or in combination with chemotherapy, have demonstrated efficacy in managing NSCLC with brain metastases [9]. Notably, the CheckMate 9LA trial showed that nivolumab plus ipilimumab, combined with a limited course of chemotherapy, provided a durable survival benefit across PD-L1 expression subgroups [10][11]. This regimen was well tolerated and has been approved as a first-line treatment for advanced NSCLC in multiple regions, including the United States and Europe. Age, however, remains a recognized negative prognostic factor in elderly patients with brain metastases, often influencing therapeutic decisions [10].
The aim of this case report is to highlight the successful management of stage IVB NSCLC with brain metastasis in elderly, comorbid patient, treated with nivolumab plus ipilimumab in combination with chemotherapy per the CheckMate 9LA protocol.
CASE REPORT
An 83-year-old woman, non-smoker with a medical history of hypertension, diabetes mellitus, and atrial fibrillation, was referred to the oncology clinic for the evaluation of a pulmonary lesion. In August 2021, following recovery from a COVID-19 infection, a chest computed tomography scan revealed a 3.2 cm mass in the left upper lobe apex, raising suspicion for malignancy (Fig. 1). This incidental finding prompted further oncological analysis. The patient reported symptoms of anxiety and depression. Upon physical examination, the patient’s temperature was 36.8 °C, heart rate 78 bpm, respiratory rate 19 bpm, blood pressure 163/94 mmHg, and oxygen saturation 92%. She experienced pleuritic chest pain (numeric pain rating score 4), shortness of breath, and easy fatigability. Her ECOG performance status was 1, primarily due to age and comorbidities. However, no problems with the central nervous system were observed.
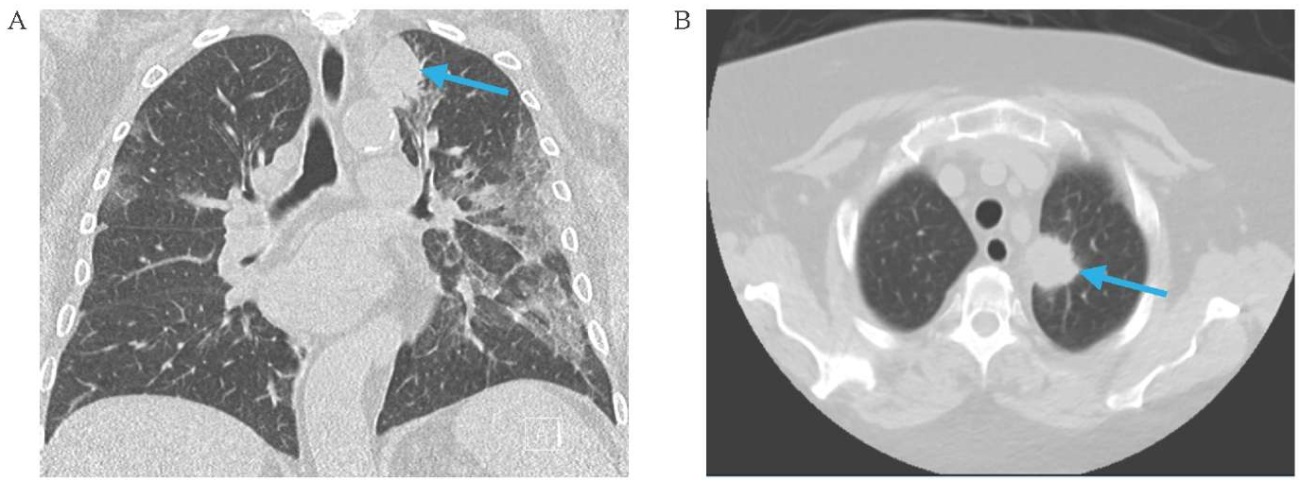
FIG. 1. Chest computed tomography at the time of initial evaluation (7th of August 2021).
A. Frontal image.
B. Axial image.
Note: left apical mass measuring 3.2 cm (arrow).
РИС. 1. Компьютерная томография органов грудной клетки на момент диагностики опухоли (7 августа 2021 г.).
А. Фронтальная проекция.
B. Аксиальная проекция.
Примечание: в верхушке левого легкого визуализируется опухолевое образование размером 3,2 см (стрелка).
Due to the patient’s low engagement in the treatment process, an active diagnostic approach was initiated only seven months after the initial detected abnormalities on computed tomography.
On March 24, 2022, a computed-tomography-guided percutaneous lung biopsy confirmed a diagnosis of poorly differentiated pulmonary adenocarcinoma. Molecular analysis was negative for actionable driver mutations. PD-L1 expression was detected in 40% of tumor cells. To evaluate for distant metastases, a positron emission tomography scan was performed on March 30, 2022 (part A of Fig. 2), and the disease was staged as IVB poorly differentiated non-small cell adenocarcinoma (T4N2M1c).
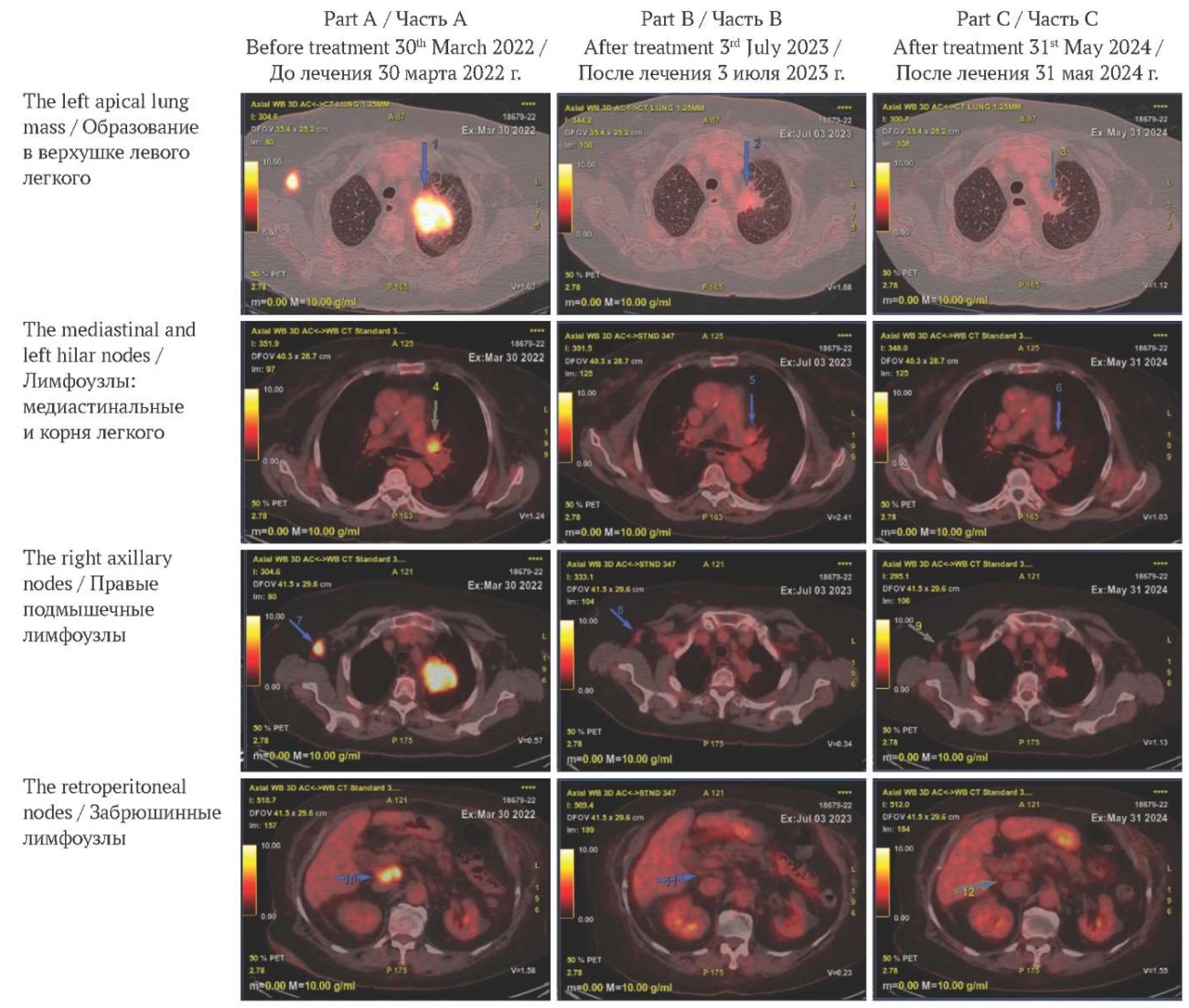
FIG. 2. Treatment-related changes on follow-up positron emission tomography scans.
Part A: before treatment. Pathological lesions with 18-fluorodeoxyglucose enhancement.
Part B: one year after the initiation of treatment. A reduction in tumor size and 18-fluorodeoxyglucose avidity.
Part C: two years after the initiation of treatment. Fully or nearly resolved 18-fluorodeoxyglucose-avid lymphadenopathy.
РИС. 2. Динамика изменений по данным позитронно-эмиссионной томографии.
Часть А: до лечения. Патологические очаги, накапливающие 18-фтордезоксиглюкозу.
Часть В: через год после начала лечения. Уменьшение размеров очагов и снижения активности захвата 18-фтордезоксиглюкозы.
Часть С: через два года после начала лечения. Полная или почти полная регрессия лимфаденопатии с накоплением 18-фтордезоксиглюкозы.
A contrast-enhanced brain magnetic resonance imaging (MRI) in April 2022 revealed a solitary ring-enhancing lesion in the left frontal lobe, considered consistent with cerebral metastasis (part A of Fig. 3). Despite the absence of neurologic symptoms, dexamethasone was administered for 14 days. No radiation therapy was initiated because of the patient’s asymptomatic status, advanced age, risk of toxicity, and emerging data supporting durable intracranial responses with immune checkpoint inhibitors.
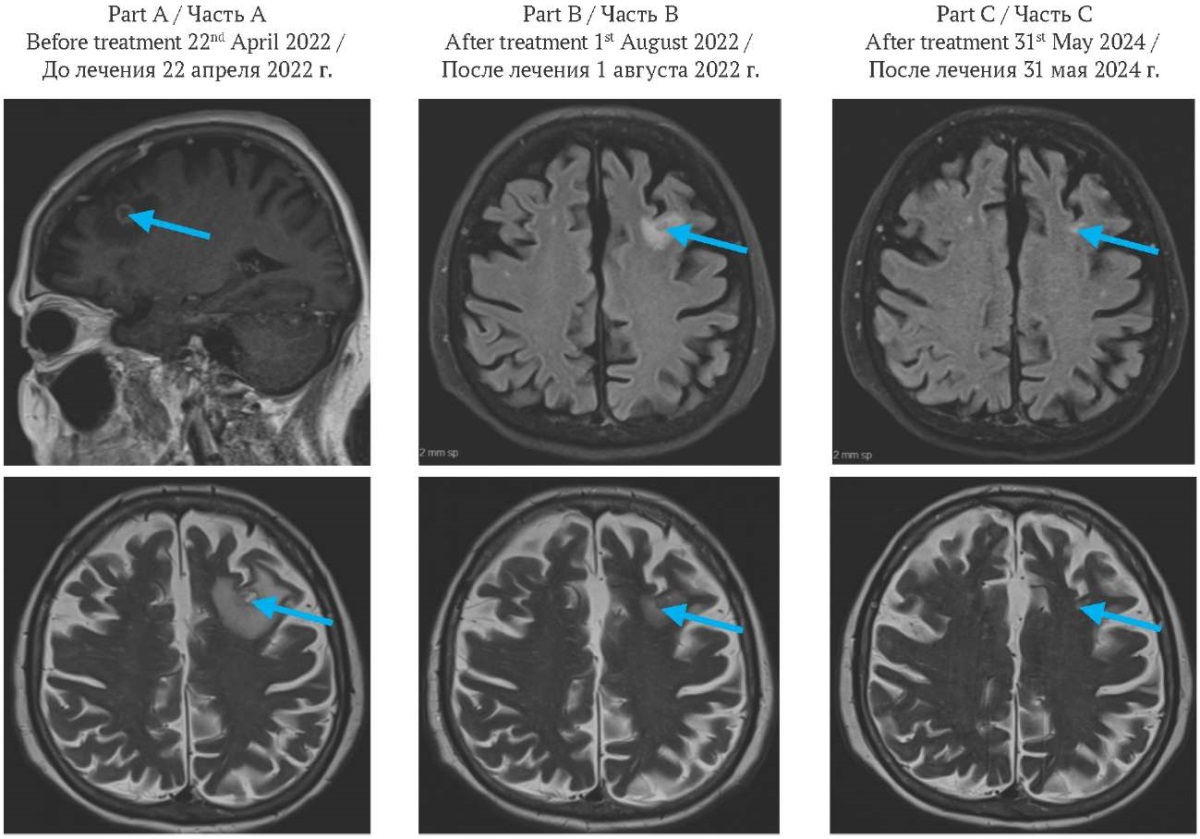
FIG. 3. Brain magnetic resonance imaging.
Part A: before treatment, frontal image (top), axial image (bottom). Solitary ring-enhancing lesion measuring 1.1×1.0 cm in the left frontal lobe (arrow), associated with marked perilesional vasogenic edema.
Part B: two months after the initiation of treatment, axial image. Solitary ring-enhancing lesion measuring 0.7 cm in the left frontal lobe (arrow), associated with reduction of perilesional vasogenic edema.
Part C: two years after the initiation of treatment, axial image. Almost resolved brain lesion and perilesional vasogenic edema (arrow).
РИС. 3. Магнитно-резонансная томография головного мозга.
Часть A: до лечения, фронтальная проекция (вверху), аксиальная проекция (внизу). Солидное кольцевидное образование размером 1,1×1,0 см в левой лобной доле (стрелка), сопровождающееся выраженным перифокальным вазогенным отеком.
Часть В: через два месяца после начала лечения, аксиальная проекция. Солидное кольцевидное образование размером 0,7 см в левой лобной доле (стрелка) с уменьшением перифокального вазогенного отека.
Часть С: через два года после начала лечения, аксиальная проекция. Практически полная регрессия очага и перифокального отека (стрелка).
Systemic therapy commenced in May 2022 with a regimen of carboplatin, pemetrexed, nivolumab, and ipilimumab, administered at a 50% dose reduction due to advanced age, in accordance with the CheckMate 9LA protocol. The second cycle was completed in June 2022. Brain MRI in August 2022 demonstrated an impressive reduction in the left frontal metastasis to 7 mm (part B of Fig. 3).
After ten cycles of immunotherapy, a positron emission tomography in July 2023 demonstrated marked reduction in both size and 18-fluorodeoxyglucose avidity of the left apical lung mass and right axillary nodes. Complete metabolic resolution was observed in the mediastinal and left hilar nodes, with near-complete resolution in the retroperitoneal nodes (Part B of Fig. 2).
By May 2024, complete resolution of both lung lesions was documented. Brain MRI showed almost complete response (part C of Fig. 3). Initial 18-fluorodeoxyglucose-avid lymphadenopathy (mediastinal, left hilar, right axillary, retroperitoneal) had fully or nearly resolved (part C of Fig. 2).
Clinical improvement paralleled the radiological response: the respiratory symptoms resolved, and the patient experienced substantial enhancement in energy, mobility, and independence. Psychological well-being also improved significantly, with restored social engagement and quality of life – key outcomes in elderly patients with multiple comorbidities. On physical examination after two years of treatment, the patient was afebrile (37.0 °C), with a heart rate of 72 bpm, respiratory rate 18 bpm, blood pressure 120/79 mmHg, and oxygen saturation of 96%. HbA1c was 6.7%. She reported minimal pain (numeric pain rating score 2), no dyspnea, and stable clinical status.
DISCUSSION
NSCLC accounts for approximately 85% of lung cancer cases and comprises several histologic subtypes, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [12]. Each subtype differs in morphology, molecular profile, and therapeutic strategies [13]. Adenocarcinoma is the most common, especially in non-smokers, while squamous cell carcinoma is strongly associated with tobacco use2. Large cell carcinoma, though less prevalent, is typically poorly differentiated and aggressive. Advances in molecular diagnostics have facilitated the identification of targetable mutations and biomarkers, such as EGFR (epidermal growth factor receptor), ALK (anaplastic lymphoma kinase), and PD-L1, enabling individualized treatment approaches [14]. Management of NSCLC may include surgery, radiotherapy, chemotherapy, targeted agents, and ICIs, depending on disease stage and biomarker status [15].
This case report describes an elderly patient with stage IVB poorly differentiated lung adenocarcinoma and brain metastasis who achieved near-complete remission without brain surgery or radiotherapy. Historically, metastatic NSCLC has been associated with poor prognosis and with a median survival of around one year [16]. However, the advent of immune checkpoint inhibitors has ushered in a new era in lung cancer treatment, significantly improving overall survival [17].
Immunosenescence may attenuate immune responses in older adults, potentially reducing ICIs efficacy [18]. Nevertheless, analyses of clinical trial data by the U.S. Food and Drug Administration indicate that patients aged ≥65 years, including those ≥75, derive similar survival benefits from ICIs as younger patients [18]. This case confirms the superiority of ICI monotherapy or chemoimmunotherapy over chemotherapy alone in terms of both survival and toxicity.
The combination of nivolumab and ipilimumab with two cycles of chemotherapy, as in the CheckMate 9LA protocol, has demonstrated sustained survival benefit versus four cycles of chemotherapy, independent of PD-L1 expression or histology [11]. In patients with low PD-L1 expression or high disease burden, combining PD-L1 ICIs with platinum-based doublet chemotherapy has been particularly advantageous. Our patient, with PD-L1 expression <50% and brain metastasis, met the profile of a candidate likely to benefit from this combined regimen.
Although the blood-brain barrier limits systemic therapy efficacy in brain metastases, emerging evidence suggests that PD-1/PD-L1 blockade may yield intracranial responses via mechanisms not yet fully elucidated [19]. Clinical trials report that patients with asymptomatic brain metastases respond favorably to nivolumab–ipilimumab combinations, with 57% intracranial benefit and 71.9% 3-year overall survival [20][21]. While we could not assess PD-L1 expression in the brain lesion, the radiographic and clinical response suggests comparable or higher PD-L1 levels. Data from the CheckMate 9LA trial reinforce the role of immunotherapy in managing NSCLC with brain metastases, highlighting its potential to improve outcomes in complex clinical scenarios.
CONCLUSION
This case highlights the potential of combination immunotherapy and chemotherapy, per the CheckMate 9LA protocol, to achieve durable remission in advanced NSCLC with brain metastasis in elderly, comorbid patients. A personalized approach combining chemotherapy and ICIs, adjusted for age and clinical status, resulted in a near-complete response and significant improvement in quality of life. Further research and prospective clinical trials are essential to define optimal therapeutic strategies for similar high-risk patient populations.
AUTHORS CONTRIBUTIONS
Ashraf ALakkad made a major contribution to the development of the concept of the article with writing and editing the case report. Aref Chehal, Aly A. Razek and Yazan Z. Alabed contributed to the interpretation of clinical data, critically reviewed the manuscript, and approved the final version for publication. Hazem M. Almasarei was responsible for the radiological analysis and its interpretation in the article. Aref Chehal, and Hamda Alkaabi helped put the manuscript together. All the authors approved the final version of the article.
ВКЛАД АВТОРОВ
Ашраф Алаккад внес значительный вклад в разработку концепции статьи, а также в написание и редактирование текста. А. Чехал, А.А. Разек и Я.З. Алабед участвовали в интерпретации клинических данных, провели критический анализ рукописи и одобрили ее окончательную версию для публикации. Х.М. Алмасарей был ответственен за радиологический анализ и его интерпретацию в статье. А. Чехал и Х. Алькааби участвовали в подготовке текста. Все авторы одобрили окончательную версию статьи.
Ethics statements. Consent statement. The patient consented to the publication of the article “Complete remission in an elderly patient with non-small cell lung cancer and brain metastasis using immunotherapy plus chemotherapy: a clinical case” in the “Sechenov Medical Journal”.
Conflict of interests. The authors declare that there is no conflict of interests.
Financial support. The study was not sponsored (own resources).
Соблюдение этических норм. Заявление о согласии. Пациентка дала согласие на публикацию представленной статьи «Полная ремиссия у пожилого пациента с немелкоклеточным раком легкого и метастазом в головной мозг при лечении иммунотерапией и химиотерапией: клинический случай» в журнале «Сеченовский вестник».
Конфликт интересов. Авторы заявляют об отсутствии конфликта интересов.
Финансирование. Исследование не имело спонсорской поддержки (собственные ресурсы).
1. Thoracic Tumours. WHO classification of tumours, 5th Edition, Volume 5. International Agency for Research on Cancer. 2021. ISBN 978-92-832-4506-3
2. Belloum Y. Circulating tumor cells (CTCs) and circulating cell-free tumor DNA (ctDNA) as blood-based biomarkers for managing non-small cell lung cancer patients [dissertation]. Hamburg: University of Hamburg; 2023. https://ediss.sub.uni-hamburg.de/handle/ediss/10671 (access date: 12.11.2024).
References
1. Rodríguez-Cid J.R., Chards S.C., González-Espinoza I.R., et al. A comparative study of immunotherapy as second-line treatment and beyond in patients with advanced non-small-cell lung carcinoma. Lung Cancer Manag. 2021 Mar 11; 10(3); LMT47. https://doi.org/10.2217/lmt-2020-0027. PMID: 34408789
2. Leone G.M., Candido S., Lavoro A., et al. Clinical relevance of targeted therapy and immune-checkpoint inhibition in lung cancer. Pharmaceutics. 2023 Apr; 15(4): 1252. https://doi.org/10.3390/pharmaceutics15041252. Epub 2023 Apr 16. PMID: 37111737
3. Chen P., Liu Y., Wen Y., et al. Non-small cell lung cancer in China. Cancer Commun (Lond). 2022 Oct; 42(10): 937-970. https://doi.org/10.1002/cac2.12359. Epub 2022 Sep 8. PMID: 36075878
4. Nicholson A.G., Tsao M.S., Beasley M.B., et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022 Mar; 17(3): 362–387. https://doi.org/10.1016/j.jtho.2021.11.003. Epub 2021 Nov 20. PMID: 34808341
5. Jiang K., Parker M., Materi J., et al. Epidemiology and survival outcomes of synchronous and metachronous brain metastases: a retrospective population-based study. Neurosurg Focus. 2023 Aug; 55(2): E3. https://doi.org/10.3171/2023.5.FOCUS23212. PMID: 37527669
6. Wang Q., Li J., Liang X., Zhan Q. Improved survival with surgical treatment of primary lung lesions in non-small cell lung cancer with brain metastases: a propensity-matched analysis of Surveillance, Epidemiology, and End Results database. Front Oncol. 2022 Jul; 12: 888999. https://doi.org/10.3389/fonc.2022.888999. PMID: 35936705
7. Schmidt M., Nitz U., Reimer T., et al. Adjuvant capecitabine versus nihil in older patients with node-positive/high-risk nodenegative early breast cancer receiving ibandronate – The ICE randomized clinical trial. Eur J Cancer. 2023 Nov; 194: 113324. https://doi.org/10.1016/j.ejca.2023.113324. Epub 2023 Sep 7. PMID: 37797387
8. Huang Q., Liu L., Xiao D., et al. CD44+ lung cancer stem cell-derived pericyte-like cells cause brain metastases through GPR124- enhanced trans-endothelial migration. Cancer Cell. 2023 Sep; 41(9): 1621–1636.e8. https://doi.org/10.1016/j.ccell.2023.07.012. Epub 2023 Aug 17. PMID: 37595587
9. Nishino M., Soejima K., Mitsudomi T., et al. Brain metastases in oncogene-driven non-small cell lung cancer. Transl Lung Cancer Res. 2019 Nov; 8(Suppl 3): S298–S307. https://doi.org/10.21037/tlcr.2019.05.15. PMID: 31857953
10. Sun C., Zhou F., Li X., et al. PD-1/PD-L1 inhibitor combined with chemotherapy can improve the survival of non-small cell lung cancer patients with brain metastases. Onco Targets Ther. 2020 Dec; 13: 12777–12786. https://doi.org/10.2147/OTT.S286600. PMID: 33363383
11. Reck M., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021 Oct; 6(5): 100273. https://doi.org/10.1016/j.esmoop.2021.100273. Epub 2021 Oct 1. Erratum in: ESMO Open. 2021 Dec; 6(6): 100345. https://doi.org/10.1016/j.esmoop.2021.100345. PMID: 34607285
12. Tai Q., Zhang L., Hu X., et al. Clinical characteristics and treatments of large cell lung carcinoma: a retrospective study using SEER data. Transl Cancer Res. 2020 Mar; 9(3): 1455–1464. https://doi.org/10.21037/tcr.2020.01.40. PMID: 35117493
13. Zhang S. Diagnostic Imaging of Lung Cancers. Springer. 2024: 103–109. https://doi.org/10.1007/978-981-99-6815-2. ISBN978- 981-99-6814-5
14. Martin-Deleon R., Teixido C., Lucena C.M., et al. EBUS-TBNA cytological samples for comprehensive molecular testing in nonsmall cell lung cancer. Cancers (Basel). 2021 Apr; 13(9): 2084. https://doi.org/10.3390/cancers13092084. PMID: 33923116
15. Rodak O., Peris-Díaz M.D., Olbromski M., et al. Current landscape of non-small cell lung cancer: epidemiology, histological classification, targeted therapies, and immunotherapy. Cancers (Basel). 2021 Sep; 13(18): 4705. https://doi.org/10.3390/cancers13184705. PMID: 34572931
16. John T., Sakai H., Ikeda S., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in advanced non-small cell lung cancer: a subanalysis of Asian patients in CheckMate 9LA. Int J Clin Oncol. 2022 Apr; 27(4): 695–706. https://doi.org/10.1007/s10147-022-02120-0. Epub 2022 Feb 19. PMID: 35182247
17. Socha J., Rychter A., Kepka L., et al. Management of brain metastases in elderly patients with lung cancer. J Thorac Dis. 2021 May; 13(5): 3295–3307. https://doi.org/10.21037/jtd-2019-rbmlc-05. PMID: 34164222
18. Shalata W., Yakobson A., Dudnik Y., et al. Multi-center real-world outcomes of nivolumab plus ipilimumab and chemotherapy in patients with metastatic non-small-cell lung cancer. Biomedicines. 2023 Aug; 11(9): 2438. https://doi.org/10.3390/biomedicines11092438. PMID: 37760878
19. Saxena P., Singh P.K., Malik P.S., Singh N. Immunotherapy alone or in combination with chemotherapy as first-line treatment of non-small cell lung cancer. Cancer Treat Oncol. 2020 Jul 27;21(8):69. https://doi.org/10.1007/s11864-020-00768-2. Erratum in: Curr Treat Options Oncol. 2020 Sep 12;21(11):91. https://doi.org/10.1007/s11864-020-00789-x. PMID: 32720019
20. Shiravand Y., Khodadadi F., Kashani S.M.A., et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022 Apr; 29(5): 3044–3060. https://doi.org/10.3390/curroncol29050247. PMID: 35621637
21. Tawbi H.A., Forsyth P.A., Algazi A., et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018 Aug; 379(8): 722–730. https://doi.org/10.1056/NEJMoa1805453. PMID: 30134131
About the Authors
A. ChehalUnited Arab Emirates
Aref Chehal, MD, Consultant, Oncology and Hematology Department; Adjunct Professor of Medicine and Oncology
Abu Dhabi, PO Box 11001
Ajman, PO Box 4184
A. ALakkad
United Arab Emirates
Ashraf ALakkad, MD, Internist, Department of Internal Medicine, Chair of Antimicrobial Stewardship Program
Mohamed Khalaf, Madinat Zayed, MZW8, Abu Dhabi, PO Box 50018
H. Alkaabi
United Arab Emirates
Hamda Alkaabi, MD, medical resident, Department of Internal Medicine
Abu Dhabi, PO Box 11001
A. A. Razek
United Arab Emirates
Aly A. Razek, MD, Consultant radiation oncologist, Chief of Department of Radiation Oncology
Al Bahia, Exit 39, Sheik Al Maktoom Road, Abu Dhabi, PO Box 5882
Y. Z. Alabed
United Arab Emirates
Yazan Z. Alabed, MD, PhD, Consultant Nuclear Medicine, Chief of Department of Nuclear Medicine and PET/CT unit
Al Bahia, Exit 39, Sheik Al Maktoom Road, Abu Dhabi, PO Box 5882
H. M. Almasarei
United Arab Emirates
Hazem M. Almasarei, MD, Consultant diagnostic and interventional radiology, Department of diagnostic and interventional radiology
Mohamed Khalaf, Madinat Zayed, MZW8, Abu Dhabi, PO Box 50018
Supplementary files
|
|
1. Graphic abstract | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(301KB)
|
Indexing metadata ▾ | |
Review
Sechenov Medical Journal. Editor's checklist for this article you can find here.
Журнал «Сеченовский вестник» |
| Sechenov Medical Journal |
Рецензии на рукопись |
| Peer-review reports |
Название / Title | Полная ремиссия у пожилой пациентки с немелкоклеточным раком легкогои метастазом в головной мозг при лечении иммунотерапией ихимиотерапией: клинический случай / Complete remission in an elderly patient with non-small cell lung cancer and brain metastasis using immunotherapy plus chemotherapy: a clinical case |
Раздел / Section
| ОНКОЛОГИЯ/ONCOLOGY
|
Тип / Article | Клинический случай / Clinical case
|
Номер / Number | 1192
|
Страна/территория / Country/Territory of origin | Объединенные Арабские Эмираты / United Arab Emirates |
Язык / Language | Английский / English
|
Источник / Manuscript source | Инициативная рукопись / Unsolicited manuscript |
Дата поступления / Received | 25.02.2025 |
Тип рецензирования / Type ofpeer-review | Двойное слепое / Double blind |
Язык рецензирования / Peer-review language | Английский / English |
РЕЦЕНЗЕНТ А / REVIEWER A
Инициалы / Initials | 1192_А
|
Научная степень / Scientific degree | Кандидат медицинских наук / Cand. of Sci. (Medicine)
|
Страна/территория / Country/Territory | Россия / Russia
|
Дата рецензирования / Date of peer-review | 21.04.2025
|
Число раундов рецензирования / Number of peer-review rounds | 2 |
Финальное решение / Final decision | принять к публикации / accept
|
ПЕРВЫЙ РАУНД РЕЦЕНЗИРОВАНИЯ / FIRST ROUND OF PEER-REVIEW
This case report is a promising example of how personalized, multidisciplinary care can lead to remarkable outcomes in challenging clinical scenarios. The manuscript provides a well-rounded discussion of the role of immune checkpoint inhibitors (ICIs) in non-small cell lung cancer (NSCLC), particularly in the context of brain metastases. It references recent clinical trials and guidelines, supporting the use of ICIs in combination with chemotherapy.
However, there are some remarks regarding this work that could enhance readers' understanding.
- The article presents MRI images from 2022 and 2024 to demonstrate the reduction and resolution of the brain metastasis. However, there is a noticeable inconsistency in the slices shown for these two time points. The 2022 images clearly depict the metastasis in the left frontal lobe, while the 2024 images do not provide a direct comparison in the same plane or slice.
- The article includes many images related to lung examinations (CT and PET scans). While these images are valuable for illustrating the patient's response to treatment, their sheer volume may overwhelm readers and detract from the key findings.
- Many of these images could be consolidated or summarized in a single composite figure, highlighting the most critical changes over time. This would streamline the presentation and make it easier for readers to focus on the most relevant data.
- The article mentions improvement in the patient's psychological symptoms and personality but does not provide detailed information on her overall quality of life during and after treatment. This is an important consideration, especially in elderly patients with multiple comorbidities.
- The article could have discussed why radiation therapy was not pursued, especially given its traditional role in managing brain metastases. A brief explanation of the rationale for avoiding radiation would have been helpful.
- While the patient's survival is impressive, the article could have provided more context by comparing her outcome to historical data or clinical trial results for similar patients treated with standard therapies.
Recommendation after the first round of peer-review: minor revision.
ВТОРОЙ РАУНД РЕЦЕНЗИРОВАНИЯ /SECOND ROUND OF PEER-REVIEW
Changes were made to the text, but there were still some shortcomings. MRI images of the brain for 2024 were not corrected. They must match the cross-sections made in 2022. It is possible to remove them completely, so it will be better.
РЕЦЕНЗЕНТ B / REVIEWER B
Инициалы / Initials | 1192_В
|
Научная степень / Scientific degree | Доктор медицинских наук / Dr. of Sci. (Medicine)
|
Страна/территория / Country/Territory | Россия / Russia
|
Дата рецензирования / Date of peer-review | 02.04.2025
|
Число раундов рецензирования / Number of peer-review rounds | 1 |
Финальное решение / Final decision | Принять к публикации после небольшой доработки/ Minor revision
|
ПЕРВЫЙ РАУНД РЕЦЕНЗИРОВАНИЯ / FIRST ROUND OF PEER-REVIEW
Scientific quality: Grade B: Good
Language quality: Grade B (Minor language polishing)
Re-review: No
The article submitted for review is a description of a clinical case, the relevance of which is associated with new data on the effectiveness of a certain chemotherapy regimen in a patient with non-small cell lung cancer. This clinical observation and its description do not contradict ethical standards. The materials and methods correspond to the stated goal. The conclusion is justified and corresponds to the obtained result. The terminology used in the text of the article is generally accepted. Literary sources are valid and correspond to the described clinical case. The text of the work does not require significant editing, the style of presentation and the level of English are satisfactory.
The authors should pay attention to the captions to the figures - the presented scans of computer tomograms are designated sagittal, while axial sections are depicted. After minimal editing the manuscript can be published.
РЕКОМЕНДАЦИИ НАУЧНЫХ РЕДАКТОРОВ ЖУРНАЛА / RECOMMENDATIONS
OF THE SCIENTIFIC EDITORS OF THE JOURNAL
Title
- Title should be no more than 150 characters with spaces. Avoid abbreviations in the title (such NSCLC, ICI).
Article highlights
- Add the ‘Article highlights’ section that contains 3 to 5 key messages.
Abstract
- Abstract should contain no more than 170 words. (Now the abstract has 262 words.)
Introduction
- Clearly define the aim of the clinical case. The aim should be placed in the end of ‘Introduction’ section.
The main text
- Add data regarding physical examination at admission:
- Blood pressure
- Heart rate, pulse
- Blood glucose level or glycated hemoglobin
- Respiratory rate
- Blood oxygen saturation
- Describe pain (constant or when breathing, visual analogue scale score)
- Add description of the tumor and metastasis (size and localization BEFORE AND AFTER treatment) in the main text, not only in figures description
- Add description of lymphatic nodes (size and localization BEFORE AND AFTER treatment) in the main text, not only in figures description
- As patient with diabetes and hypertension has taken dexamethasone and cardiotoxic medication, provide data regarding physical examination after two years of treatment (Blood pressure, Heart rate, pulse, Blood glucose level / glycated hemoglobin).
Figures
- Provide the same slices of the brain MRI.
- Provide ALL figures in separate files in high quality.
- Figures should be numbered in Arabic numerals, have a legend (Figure 1 … (the name)) and have a link in the text (fig. 1). Only one legend is provided for each multi-panel figure consisting of graphs that depicts data of the same theme (e.g., Figure 1 Magnetic resonance imaging of the brain. A: …; B: …).
Technical requirements
- Add information about all authors (full surname and name, academic degree and title (if any), position, place of work (or study), ORCID).
- For corresponding author provide full first and family (sur)names, abbreviated title (e.g., MD, PhD), affiliated institute’s name and complete postal address (including zip code).
- Specify authors’ contribution.
- Provide section ‘Financial support’.
- Provide ‘List of abbreviation’ before the main text.
- All abbreviations used in the article should be decrypted after they were firstly mentioned.
References
- In the text of the article, bibliographic references are given in Arabic numerals in square brackets (e.g., [1-3]).
- The ‘REFERENCES’ section lists the references in the Vancouver style. Check the authors guidelines on the Sechenov Medical Journal website: https://www.sechenovmedj.com/jour/about/submissions#authorGuidelines
- Provide DOI and PMID for each reference. In case the reference has no DOI or PMID placed it in footnotes and removed from bibliography.
- The reference 6 should be placed in footnotes and removed from bibliography. Provide in footnotes the web-link and date of access.
- For reference 13 provide ISBN.
- The reference 14 should be placed in footnotes and removed from bibliography. Provide in footnotes the web-link and date of access.
- Provide journal name for references 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 15, 16, 17, 19, 20, 21, 22, 23.